Akebia Therapeutics (AKBA) has announced plans to cut down its headcount by 42% by the end of June. The move comes after the U.S. Food and Drug Administration rejected the company’s new drug application for vadadustat to treat anemia due to chronic kidney disease (CKD).
Invest with Confidence:
- Follow TipRanks' Top Wall Street Analysts to uncover their success rate and average return.
- Join thousands of data-driven investors – Build your Smart Portfolio for personalized insights.
The biopharmaceutical company engages in the development and commercialization of therapeutics for patients with kidney diseases. Shares of AKBA fell 6.3% at the time of writing.
Akebia now plans to focus on Auryxia, the trade name for ferric citrate, which is used to control serum phosphorus levels in adult patients with CKD on dialysis and for the treatment of anemia in adult patients with CKD not on dialysis.
Akebia expects a one-time hit of about $12 million, related to severance, non-cash stock-based compensation expense, and healthcare and related benefits in Q2. Also, it projects layoffs to result in about a $60-65 million reduction in net cash.
Wall Street’s Take
Following the news, H.C. Wainwright analyst Ed Arce maintained a Hold rating on Akebia.
Arce said, “Given management had estimated its cash resources to be sufficient to fund the previous operating plan (with the assumption that vadadustat would be approved by the FDA by March 29) through at least the next 12 months, we estimate this workforce reduction to extend Akebia’s cash runway by about six months.”
“As Akebia and its partner Otsuka continue to engage with the EMA on its ongoing MAA of vadadustat, we regard vadadustat’s potential approval in Europe by YE22 as Akebia’s next major catalyst,” the analyst added.
Based on five Holds and one Sell, the stock has a Hold consensus rating. Akebia’s average price forecast of $2 implies 267.7% upside potential from current levels. Shares have lost 81.7% over the past year.
Insider Trading
Based on the recent corporate insider activity, sentiments seem Very Negative. This means that over the past quarter there has been an increase in insiders selling their shares of AKBA.
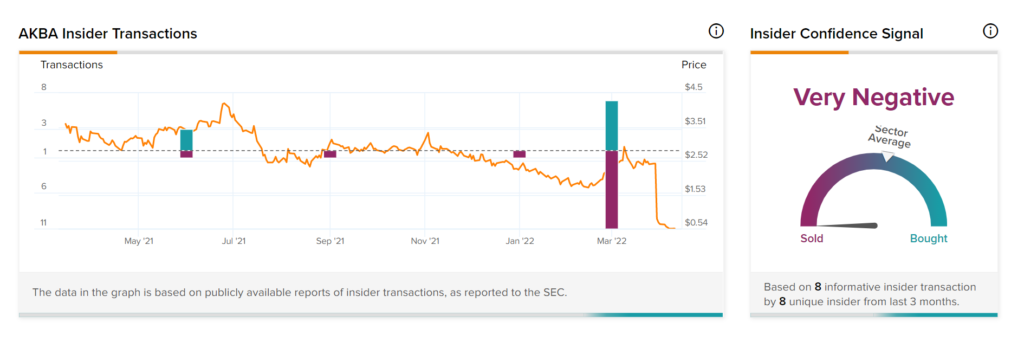
Takeaway
FDA’s rejection has come as a shocker for investors who are now likely to stay on the sidelines until the company proves its worth with some positive update on vadadustat. Based on negative signals of corporate insiders and hedge funds, it is visible that they have little confidence in the stock.
Download the TipRanks mobile app now
Discover new investment ideas with data you can trust.
Read full Disclaimer & Disclosure
Related News:
Will Robinhood’s Lightning-Fast Transactions Aid Growth?
Why Did Vapotherm Nosedive on Thursday?
Accenture Solidifies its Sustainability Offerings









