Shares of Aeglea BioTherapeutics, Inc. (NASDAQ: AGLE) gained almost 28% during the extended trading session on April 11. AGLE shares rose after the clinical-stage biotechnology company revealed additional data from the PEACE Phase 3 study related to the study of pegzilarginase for the treatment of the rare, inherited and progressive disease Arginase 1 Deficiency (ARG1-D).
Maximize Your Portfolio with Data Driven Insights:
- Leverage the power of TipRanks' Smart Score, a data-driven tool to help you uncover top performing stocks and make informed investment decisions.
- Monitor your stock picks and compare them to top Wall Street Analysts' recommendations with Your Smart Portfolio
Aeglea BioTherapeutics designs and develops human enzyme therapeutics for the treatment of patients with rare metabolic diseases with limited treatment options.
Prior Approvals
Pegzilarginase, the company’s lead product candidate, is a recombinant human Arginase 1 enzyme. It is in the Phase III PEACE trial to evaluate the safety and efficacy for the treatment of Arginase 1 deficiency. So far, pegzilarginase has received both “Rare Pediatric Disease” and “Breakthrough Therapy” designations.
Arginase 1 Deficiency (ARG1-D) is a rare inherited metabolic disorder marked by high levels of arginine, a building block of protein. Patients suffering from ARG1-D develop severe spasticity-related mobility limitations, seizures, developmental delay, intellectual disability, and early mortality.
Earlier, in December 2021, Aeglea announced positive topline data from its PEACE Phase 3 clinical trial for pegzilarginase in patients with Arginase 1 Deficiency. The data revealed that the primary endpoint was met and there was a significant 76.7% reduction in mean plasma arginine in pegzilarginase-treated patients versus placebo.
Supporting Data
The currently-disclosed positive data was disclosed during a poster presentation at the Society for Inherited Metabolic Disorders (SIMD) Annual Meeting held in Orlando.
In the individual patient-based outcome analysis, 11 patients (65%) treated with pegzilarginase reached or exceeded prespecified response criteria for at least one mobility assessment versus four patients (44%) that were on placebo.
Further, eight patients (47%) met or exceeded prespecified clinical response criteria for at least two of the mobility outcomes, in comparison to no patients receiving placebo.
CEO Comments
Aeglea CEO, Anthony G. Quinn, commented, “Our clinical development program for pegzilarginase has provided the first quantitative insights into the disease burden of ARG1-D and sheds new light on both the severity of the disease and the devastating effect of uncontrolled arginine levels in these patients.”
He further added, “The data from PEACE show the ability of pegzilarginase to markedly improve arginine control and its impact on a broad range of disease-related abnormalities. We are pleased with these results and believe pegzilarginase has the potential to change the lives of the patients and families living with ARG1-D.”
Wall Street’s Take
Turning to Wall Street, the analyst consensus is also optimistic about Aeglea Biotherapeutics, with a Strong Buy rating based on six unanimous Buys. The average Aeglea Biotherapeutics price target of $13.20 indicates an upside potential of a whopping 443.21%.
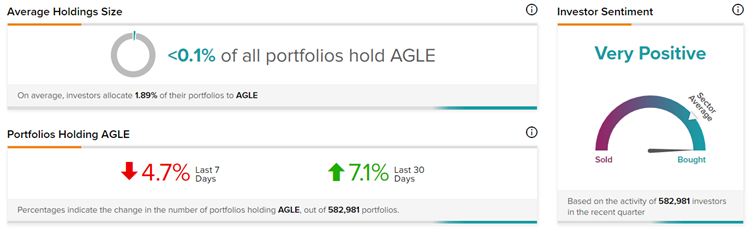
Investors Weigh In
TipRanks’ Stock Investors tool shows that investors currently have a Very Positive stance on Aeglea Biotherapeutics, with 7.1% of investors increasing their exposure to AGLE stock over the past 30 days.
Take Away
Pegzilarginase, the company’s lead product candidate, has achieved promising Phase 3 data and looks like it is on its way to gaining complete approval. This could be a huge catalyst for the stock making it a multi-bagger stock with significant returns to investors.
Discover new investment ideas with data you can trust.
Read full Disclaimer & Disclosure
Related News:
Moderna Recalls 764,900 COVID-19 Doses in Europe
JetBlue Trims Flight Schedules
Novavax Receives Authorization to Inject COVID-19 Vaccine in Thailand









