Verrica Pharmaceuticals on Dec. 23 announced the resubmission of the New Drug Application (NDA) of its VP-102 used in the treatment of molluscum contagiosum to the US Food and Drug Administration (FDA).
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
Verrica’s (VRCA) move follows actions taken as requested by the FDA’s Complete Response Letter (CRL) issued in July this year. Shares of the dermatology therapeutics company lost 5% at the close on Dec. 24.
Molluscum contagiosum is a highly infectious skin disease caused by a pox virus that affects around 6 million people in the US, mainly children. VP-102, is an exclusive drug-device combination for the treatment of molluscum contagiosum. It would be marketed in the US under the brand name YCANTH upon the FDA approval.
The company has successfully completed a Phase 2 study of VP-102 for the treatment of common warts and a Phase 2 study of VP-102 for the treatment of external genital warts.
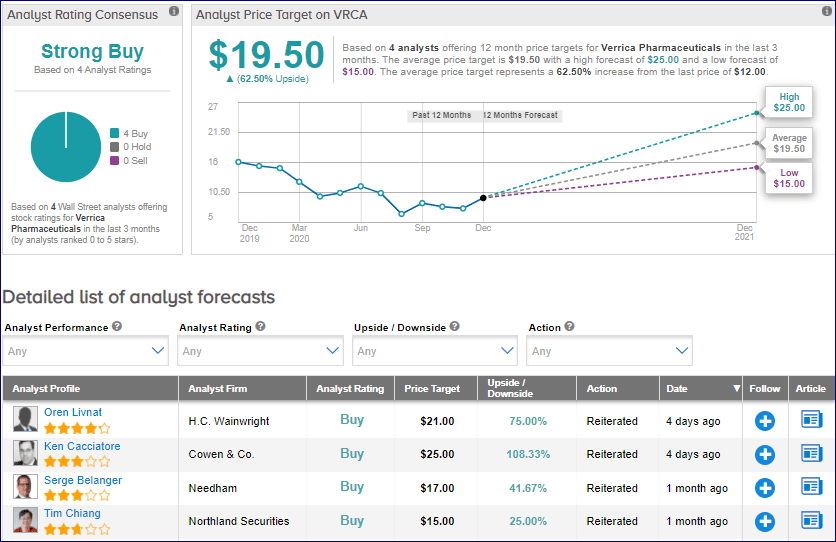
In reaction to the FDA resubmission, Cowen analyst Ken Cacciatore on Dec. 24 reiterated a Buy rating on VRCA stock with a $25 price target . The price target implies 108% upside potential.
Cacciatore expects the FDA to accept the resubmission, with a potential approval by the third quarter of 2021.
The analyst is confident that VP-102 “would fulfill a large unmet need,” as it is set to become the first FDA-approved therapy for molluscum. (See VRCA stock analysis on TipRanks)
From the rest of the Street, the stock scores an analyst consensus of a Strong Buy based on 4 unanimous Buys. The average analyst price target of $19.50 implies upside potential of about 63% to current levels. Shares have dropped 24.5% year-to-date.
Related News:
Alibaba Ups Buyback Program to $10B; Street Firmly Bullish
Village Farms Issues Statement On Share Rally; Top Analyst Sees 67% Upside
Facebook Shuts Irish Subsidiaries Amid Tax Row – Report