Moderna (NASDAQ:MRNA) announced on Tuesday that the U.S. government had awarded the pharmaceutical and biotech company $176 million to advance its bird flu vaccine development. The government has awarded this grant through its Rapid Response Partnership Vehicle (RRPV). This agreement also includes “additional options to prepare and accelerate a response to future public health threats.”
Last year, Moderna initiated a Phase 1/2 study of its mRNA-1018 bird flu vaccine in adults, covering H5 and H7 subtypes of avian influenza. The results of this study are expected later this year and will guide the company’s Phase-3 development plans. Usually, conventional flu vaccine manufacturing takes four to six months.
If the company’s Phase1/2 study of its mRNA-1018 bird flu vaccine is successful, it could strengthen its vaccine pipeline.
Why the Need for a Bird Flu Vaccine?
Earlier this year, the U.S. reported the first outbreak of H5N1 (bird flu) in dairy cattle that has since infected more than 130 herds in 12 states. Scientists are worried that exposure to the virus in poultry and dairy operations could lead to a virus mutation that spreads easily among humans, risking the start of a pandemic.
What Is the Future Price of MRNA Stock?
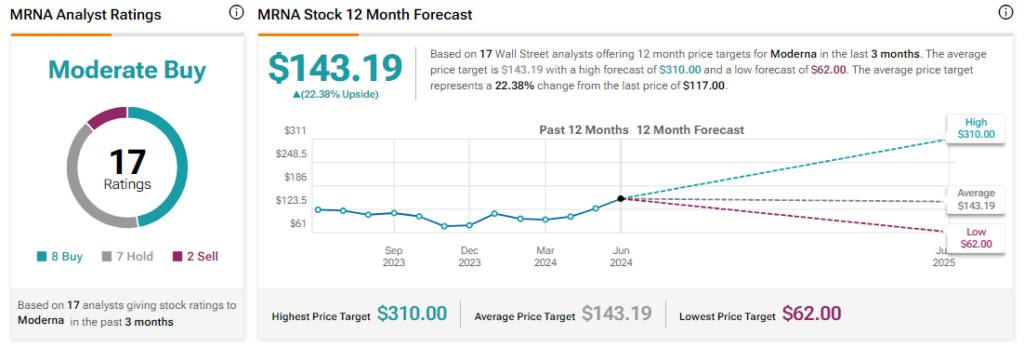
Analysts remain cautiously optimistic about MRNA stock, with a Moderate Buy consensus rating based on eight Buys, seven Holds, and two Sells. Year-to-date, MRNA has increased by more than 15%, and the average MRNA price target of $143.19 implies an upside potential of 22.4% from current levels
Questions or Comments about the article? Write to editor@tipranks.com
















