Shares in Trillium Therapeutics (TRIL) spiked 38% in Tuesday’s after-hours trading, after the immuno-oncology company announced updated data from its ongoing TTI-622 and TTI-621 dose escalation studies. The stock has now exploded by an incredible 818% year-to-date.
Don't Miss our Black Friday Offers:
- Unlock your investing potential with TipRanks Premium - Now At 40% OFF!
- Make smarter investments with weekly expert stock picks from the Smart Investor Newsletter
“We are exceedingly encouraged by the evolving profile of TTI-622, our SIRPa-IgG4 Fc fusion protein, as demonstrated in the ongoing dose escalation study in relapsed and refractory lymphomas,” said Jan Skvarka, Trillium’s CEO.
“TTI-622 is showing substantial monotherapy activity in highly pre-treated patients, with a broad therapeutic window, a rapid onset of action, and across a range of lymphoma indications. With no significant safety signals observed, we are further escalating the dose” he said.
Meanwhile TTI-621, the company’s SIRPa-IgG1 Fc fusion protein, is also showing a strong safety profile, without any dose limiting thrombocytopenia for doses up to 1.4 mg/kg. Skvarka commented: “We continue to see a monotherapy activity signal, and are further dose escalating to characterize clinical activity at higher doses.”
The CEO says that he expects TRIL to declare maximum tolerated doses or recommended phase 2 doses for both molecules either towards the end of this year or in the first half of 2021.
Abstracts for both trials have been submitted to the American Society of Hematology annual meeting, said Skvarka, with further details and additional data to be presented in December.
TTI-622 is being evaluated in a two-part phase 1a/1b study in patients with advanced relapsed or refractory lymphoma or multiple myeloma. One Grade 4 dose-limiting toxicity (DLT) was reported among six patients; with six objective responses (33%; 1 complete response, 5 partial responses) among 18 patients. Clinical responses were observed across multiple lymphoma indications. The study is currently enrolling patients at the 12 mg/kg dose level.
TTI-621 is being evaluated in a four-part phase 1 study in patients with advanced relapsed or refractory hematologic malignancies. In the ongoing Part 4, TTI-621 dosing is being escalated beyond 0.5 mg/kg in patients with cutaneous T-cell lymphoma. Preliminary data indicate the weekly infusions of up to 1.4 mg/kg are well tolerated.
One Grade 3 IRR DLT was observed at 1.0 mg/kg, with antitumor activity in the 1 mg/kg cohort including 1 partial response and 1 complete response in 6 evaluable patients; 2 patients were bridged to allogeneic transplantation. The study is currently enrolling patients at the 2.0 mg/kg dose level.
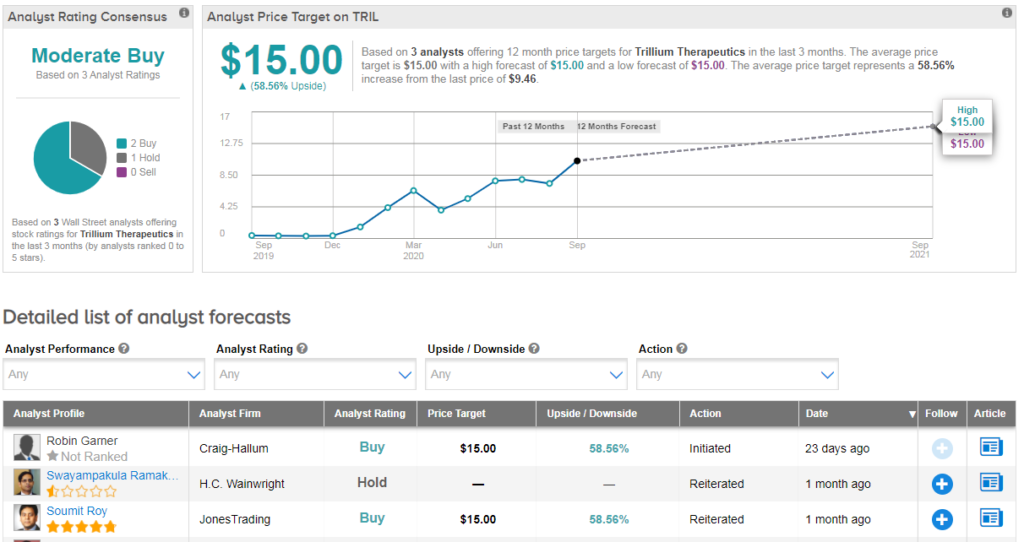
Three analysts have published recent ratings on TRIL, with 2 buy ratings vs 1 hold. Their average price target of $15 indicates 59% further upside potential lies ahead.
“Both TTI-621/622 are the only CD47-targeting drug candidates that showed multiple complete responses in low dose cohorts in monotherapy regimen – likely to improve efficacy in combination regimens and at high dose levels” cheers JonesTrading analyst Soumit Roy.
He has a buy rating on the stock and $15 price target, writing: “With increased investor interest in SIRPα-CD47 pathway, differentiated assets, cash runway into 2022 and biotech focused investors in the stock, we believe Trillium is well positioned with multiple stock inflection points in the next six to nine months.” (See TRIL stock analysis on TipRanks).
Related News:
Trillium: Recent Weakness Presents a Buying Opportunity, Says Analyst
Morgan Stanley Turns Bullish On Eli Lilly, Lifts PT
AstraZeneca’s Covid-19 Trial Paused After Adverse Reaction; Stock Falls 8%