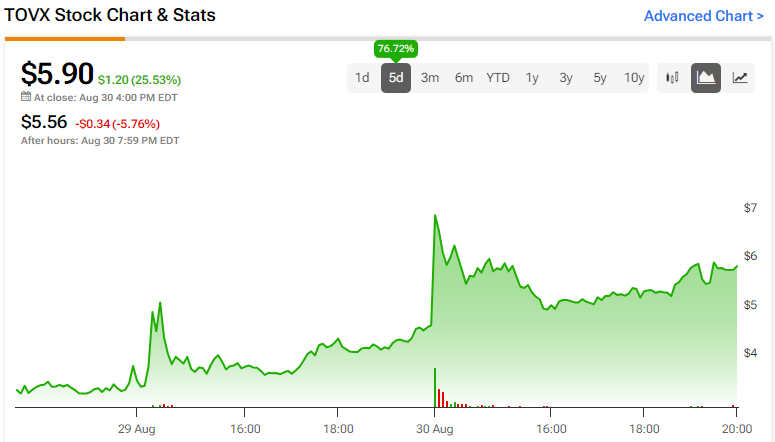
Theriva Biologics (TOVX) is making noise in the field of immuno-oncology, supported by a promising pipeline. The stock is up 79% in the past few days on the news the company’s lead clinical-stage candidate, VCN-01, received a Rare Pediatric Drug Designation (RPDD) from the FDA to treat pediatric patients with retinoblastoma, an eye cancer in children, highlighting the drug’s potential. Furthermore, Theriva recently reported Q2 earnings that exceeded analysts’ estimates and executed a reverse stock split. It is still early for Theriva, but investors interested in the high-risk, high-reward game of finding biotech diamonds in the rough might want to keep this one on their radar.
Invest with Confidence:
- Follow TipRanks' Top Wall Street Analysts to uncover their success rate and average return.
- Join thousands of data-driven investors – Build your Smart Portfolio for personalized insights.
Theriva’s Promising Pipeline
Theriva is a clinical-stage immuno-oncology company that concentrates on creating oncolytic viruses to treat solid tumors, providing patients with potential trailblazer treatments for difficult-to-treat cancers. The company’s subsidiary, Theriva Biologics, S.L., is making advancements on a new oncolytic adenovirus platform intended for delivery to trigger tumor cell death and facilitate the patient’s immune system response. Its primary clinical-stage candidates include VCN-01, SYN-004, and SYN-020.
The U.S. FDA recently granted VCN-01 the Rare Pediatric Disease Designation (RPDD), the leading clinical candidate for treating retinoblastoma in children. If Theriva secures the final approval for the Biologics License Application for VCN-01, it may qualify for a Priority Review Voucher. This voucher can expedite the review process for future marketing applications, or it could be sold or transferred.
Furthermore, the FDA has granted VCN-01 a Fast Track Designation (FTD) for treating Pancreatic duct adenocarcinoma (PDAC). Currently, the company is conducting dosing for VCN-01 to provide it as a first-line therapy for newly diagnosed metastatic PDAC patients. The expectation is to complete patient enrollment in this trial by the third quarter of 2024.
It is also worth noting the progress of SYN-004. The treatment targeted at preventing gastrointestinal damage is in a Phase 1b/2a trial. Dosing and safety follow-ups have been completed for allogeneic hematopoietic cell transplant (HCT) recipients.
Analysis of Theriva’s Recent Financial Results
The company recently announced its second-quarter results for 2024. No revenue was reported for the quarter, and there was a notable decrease in both general and Administrative expenses and research and development costs compared to the same period in 2023. Although there was a drop in clinical trial costs, expenditures were slightly offset by increased expenses related to the Phase 1b/2a clinical trial of SYN-004. Earnings per share (EPS) of -$10.75 missed analysts’ estimates of -$4.75.
The company reduced the value of its goodwill from $5.5 million to $1.5 million as the market price of its common stock decreased significantly, triggering an impairment. In response, Theriva announced a reverse stock split to reduce the number of outstanding common shares from 25,131,230 to 1,005,249 to meet the per-share price requirements of the NYSE American.
At the quarter’s end, the company’s cash and cash equivalents totaled $16.6 million, compared to $23.2 million as of December 31, 2023.
Is TOVX a Buy?
It is a speculative situation appropriate only for those investors willing and able to endure a volatile ride over the next few years as the company’s lead pipeline candidates progress through the clinical testing process.
The stock is no stranger to enthusiastic runs, with the share price exceeding $275 in early 2021. It has been downward since, shedding over 95% in the past three years. The recent positive news has catalyzed a jump in price, and the stock now shows positive price momentum across all major moving averages.
Wall Street follows the company thinly. However, Maxim Group analyst Jason McCarthy recently reiterated a Buy rating on TOVX stock. Theriva Biologics is rated a moderate buy based on the most recent analyst recommendation.

Final Thoughts on TOVX
Theriva Biologics is catching the financial world’s attention with an eye-popping 79% rise in its stock price over the last week on positive news regarding the company’s lead clinical-stage candidate. However, it is an early-stage biotech, and while indications are promising, it’s important to remember investing in such companies comes with high risk and potentially high rewards. Nonetheless, the progress in its clinical trials and a promising pipeline make TOVX a stock to watch.









