Shares of TG Therapeutics spiked 12% on Feb. 5 after the US Food and Drug Administration (FDA) approved Ukoniq (umbralisib), its investigational compound, for the treatment of adult patients with relapsed or refractory marginal zone lymphoma (MZL). The stock gained another 4% in Friday’s after-hours trading.
Specifically, the FDA granted approval for the treatment of Ukoniq in MZL patients who have received at least one prior anti-CD20 based regimen and adult patients with relapsed or refractory follicular lymphoma (FL) who have received at least three prior lines of systemic therapy.
TG Therapeutics (TGTX) is a biopharma company focused on the development of novel treatments for B-cell malignancies and autoimmune diseases. The company’s two compounds, ublituximab and umbralisib (the combination of which is referred to as U2) are in late-stage clinical development for patients suffering from hematologic malignancies, with ublituximab also in clinical development for multiple sclerosis (MS).
“Today’s approval of UKONIQ marks a historic day for our Company with this being our first approval and we are extremely pleased to be able to bring our novel inhibitor of PI3K-delta and CK1-epsilon to patients with relapsed/refractory MZL and FL,” TG Therapeutics CEO Michael S. Weiss stated. “We expect to make UKONIQ available to US distributors in the next few days.”
TG Therapeutics said that the accelerated FDA approval was based on efficacy data from Ukoniq’s monotherapy, which was evaluated in two single-arm cohorts as part of the Phase 2 clinical trial, in 69 patients with MZL who received at least 1 prior therapy, including an anti-CD20 regimen, and in 117 patients with FL who received at least 2 prior systemic therapies. The Phase 2 trial is an open-label, multi-cohort study with patients receiving Ukoniq 800 mg once daily.
TGTX shares have exploded over 256% over the past year in reaction to a few favorable pipeline updates. Nonetheless, B. Riley Financial analyst Mayank Mamtani believes that the stock has more room to grow.
Mamtani lifted the stock’s price target to a Street-high of $90 (66% upside potential) from $67 and reiterated a Buy rating, citing the commercial opportunity of TGTX’s anti-CD20, ublituximab for the treatment of MS.
Based on a survey of 31 US-based MS treating physicians, the analyst is confident in “TGTX entering the RMS [relapsed multiple sclerosis] market as a disruptive competitor; implying improved market penetration & share dynamics as well as likelihood of regulatory success supporting TGTX’s mid-2021 NDA [new drug application] submission.” (See TGTX stock analysis on TipRanks)
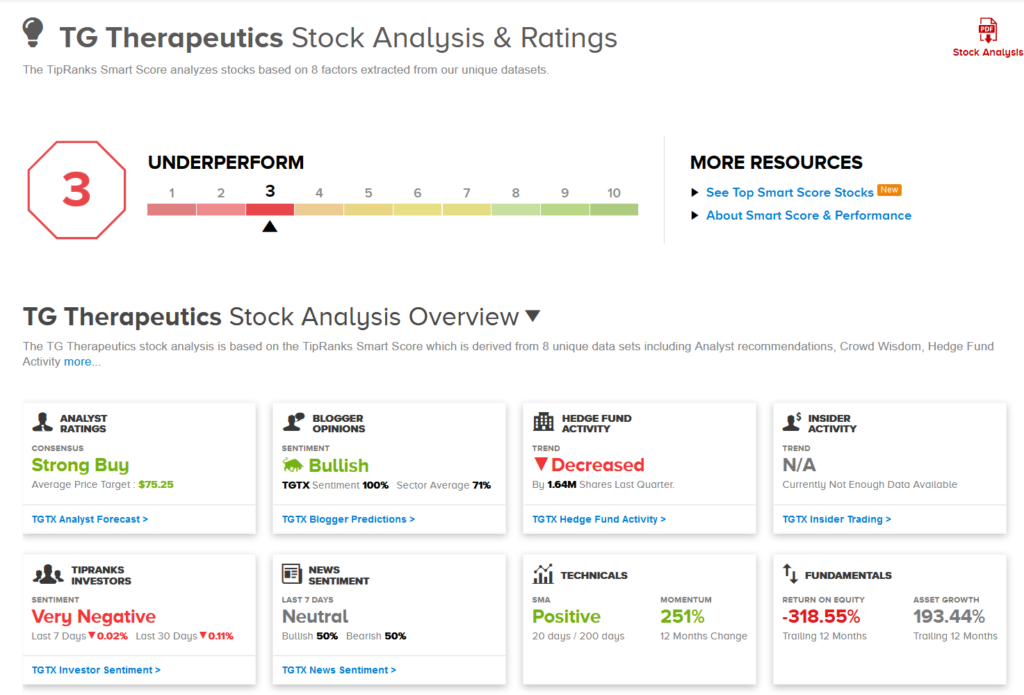
The Street mirrors Mamtani’s optimism, with a Strong Buy analyst consensus backed by 4 unanimous Buys. Despite the meteoric rise in the stock, the price target of $75.25 still implies upside potential of 39% over the coming year.
Meanwhile, TGTX gets a 3 out of 10 on TipRanks’ Smart Score system, which indicates that the stock is likely to underperform market expectations.
Related News:
J&J Files For FDA Approval Of COVID-19 Vaccine; Street is Bullish
Shoe Carnival’s 4Q Profit Outlook Tops Estimates; Shares Gain 5%
Illinois Tool’s ‘Record’ Operating Margin Drives 4Q Profit Beat
















