Teva Pharmaceuticals Europe has received marketing authorization for Seffalair and BroPair Spiromax in the European Union to treat asthma. The drugs have been approved as maintenance treatment for asthma in patients above 12 years of age.
Paul Blonk, Head of Teva (TEVA) Respiratory Europe, said, “We are excited about the European approval of Seffalair Spiromax and BroPair Spiromax, as an important goal of our respiratory franchise is to bring new treatment options to healthcare professionals who support people living with long-term conditions such as asthma.”
Blonk added, “We want to empower patients to effectively manage their condition through the medicines we provide, whilst also offering cost-effective treatments to healthcare systems.” (See Teva Pharmaceuticals stock analysis on TipRanks)
The company plans to initially launch the products in Portugal, Switzerland, Spain and the UK. According to Teva, there are about 28 million asthma patients in the European Union.
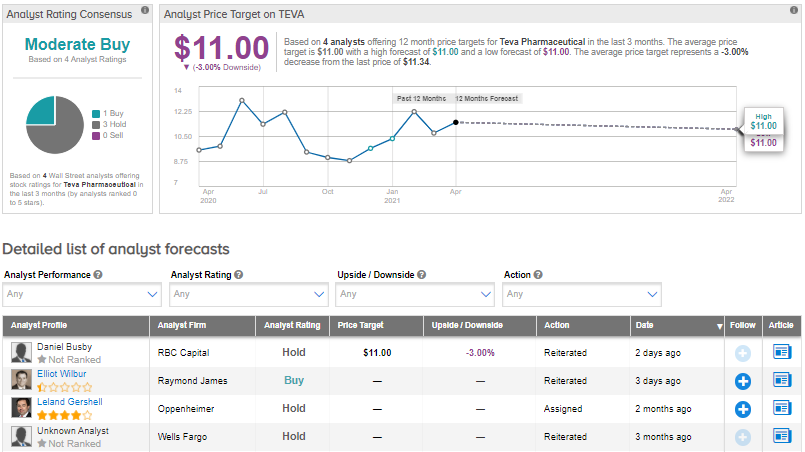
On April 5, Raymond James analyst Elliot Wilbur reiterated a Buy rating on the stock but did not assign any price target.
Wilbur commented on the weekly prescription trend, “Ajovy NBRx share has more than doubled since late February, increasing to around 25%, the highest level since May 2019.”
Overall, the consensus on the Street is that Teva is a Moderate Buy, based on 1 Buy and 3 Holds. The average analyst price target of $11 implies 3% downside potential. Shares have gained about 18.9% over the past year.
Related News:
Beyond Meat Opens First Manufacturing Facility In China
Nokia Settles Patent Dispute With Lenovo
Fox Files Suit Against Flutter Over Stake Dispute In Sports Betting Firm FanDuel



















