TCR2 Therapeutics Inc. (TCRR) has announced positive interim data from the first five patients treated in the Phase 1 portion of the TC-210 Phase 1/2 clinical trial for mesothelin-expressing solid tumors.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
All five patients showed tumor regression including two RECIST unconfirmed partial responses (one of which remains subject to independent central review) and two patients with stable disease through six months.
Translational data further demonstrated TRuC-T cell expansion and activation.
A manageable toxicity profile was observed with only one patient exhibiting TC-210-related non-hematologic grade >2 toxicity and no evidence of neurotoxicity or on-target, off-tumor toxicity.
“We are delighted that our very first dose of TC-210 induced consistent tumor regression and clinical benefit in heavily pre-treated cancer patients,” cheered CEO Garry Menzel.
“There are very few options for patients with solid tumors and those expressing mesothelin represent a significant frontier of unmet medical need. While these are early data requiring further study, we are encouraged by the potential of our TRuC-T cells as we continue to enroll and treat patients with the goal of quickly finding a recommended Phase 2 dose for TC-210” he added.
The primary objectives of the Phase 1 portion of the study are to define the safety profile of TC-210 in patients whose tumors overexpress mesothelin and to determine the recommended Phase 2 dose (RP2D). Secondary objectives include overall response rate (ORR) and disease control rate (DCR). Exploratory objectives include the assessment of expansion, tumor infiltration, and persistence of TC-210 T cells.
In the Phase 2 portion of the clinical trial, approximately 50 patients are planned to receive TC-210 at the RP2D in four groups according to their cancer diagnosis: NSCLC, ovarian cancer, malignant pleural/peritoneal mesothelioma and cholangiocarcinoma.
Each group will include ten patients, except the NSCLC group which will include 20 patients with eight patients to receive TC-210 as single agent and 12 patients to receive TC-210 in combination with a programmed cell death 1 (PD-1) blocking antibody.
Mesothelin is a cell-surface glycoprotein highly expressed in a wide range of solid tumors- and overexpression of mesothelin is associated with poorer prognosis in some cancers due to its active role in both malignant transformation and tumor aggressiveness.
Of the wide range of solid tumors expressing mesothelin, non-small cell lung cancer, ovarian cancer, mesothelioma and cholangiocarcinoma represent a significant patient population up to 80,000 in the United States alone.
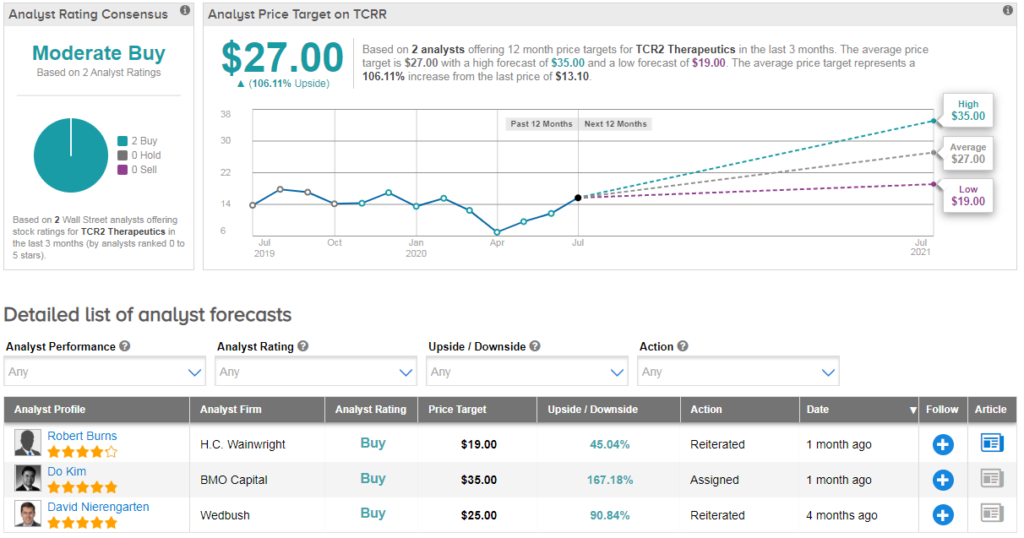
Both HC Wainwright’s Robert Burns and BMO Capital’s Do Kim have recently issued buy ratings on the stock. Their average price target of $27 indicates that shares can double from current levels. Shares are down 9% year-to-date. (See TCRR stock analysis on TipRanks).
Related News:
Gilead’s Kite Gets FDA Nod For Tecartus Blood Cancer Treatment
NuVasive Spikes 5% After-Hours On Sharp Procedure Rebound
Dr Reddy’s Scoops FDA Nod For Breakthrough Head Lice Treatment