Spectrum Pharmaceuticals announced that the FDA has agreed to the submission of the poziotinib NDA for non-small cell lung cancer (NSCLC) in previously treated patients with HER2 exon 20 insertion mutations, based on data from Cohort 2 of its Phase 2 clinical trial, ZENITH20. Despite the favorable update, shares fell 9.7% in the extended trading session on Tuesday.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
Investors were disappointed as Cohort 3 of Spectrum’s (SPPI) ZENITH20 clinical trial, which enrolled first-line NSCLC patients with EGFR exon 20 insertion mutations at 16mg once daily, did not meet its primary endpoint.
The company disclosed that it had a successful pre-NDA meeting with the FDA, which resulted in an agreement to submit an NDA for poziotinib. It confirmed with the FDA that Cohort 2 data could serve as the basis of an NDA submission and plans to submit the application in 2021. (See SPPI stock analysis on TipRanks)
Francois Lebel, Spectrum’s Chief Medical Officer, stated, “While Cohort 3 did not meet its pre-specified ORR endpoint, we are seeing evidence of clinical activity with a disease control rate (DCR) of 86% and progression free survival data of 7.2 months.”
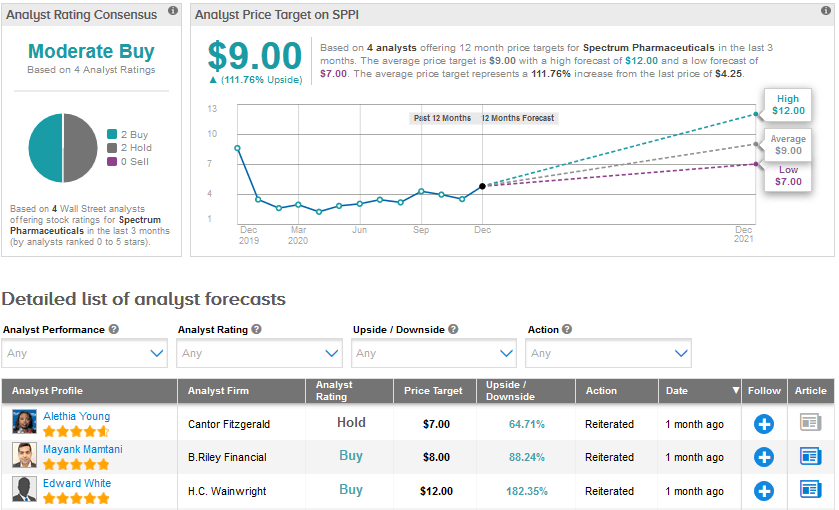
The Street is cautiously optimistic on Spectrum, with a Moderate Buy analyst consensus based on 2 Buys and 2 Holds. The average price target stands at $9, suggesting a significant upside potential of about 112% in the months ahead.
Last month, B. Riley Financial analyst Mayank Mamtani reiterated a Buy rating on Spectrum Pharma with a price target of $8 following the company’s 3Q results. The analyst feels that Spectrum’s resilient pipeline execution sets up a catalyst rich two to six months, both for poziotinib and Rolontis.
Related News:
Columbia Care Agrees to Buy Green Leaf Medical for $240M
Pfizer-BioNTech Covid-19 Vaccine Gets Cleared For Use In Europe
Moderna’s Covid-19 Vaccine Wins FDA Emergency Use Approval