A new complaint was filed against Humacyte, Inc. (HUMA) by shareholder (plaintiff) James A. Cutshall on November 18, 2024, in the U.S. District Court for the Middle District of North Carolina. The defendants in the complaint are the company, founder and CEO Laura E. Niklason, CFO Dale A. Sander, and COO Heather Prichard.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
The plaintiff alleges that he bought HUMA stock at artificially inflated prices between May 10, 2024 and October 17, 2024 (the “Class Period”). The plaintiff is now seeking compensation for his financial losses. To learn more about the lawsuit, click here.
Humacyte is focused on developing implantable bioengineered human tissues, advanced tissue constructs, and organ systems.
The filed complaint alleges that during the Class Period, the defendants misled Humacyte investors in violation of Sections 10(b) and 20(a) of the Securities Exchange Act.
Plaintiff’s Allegations
According to the complaint, Humacyte intentionally misrepresented information presented in its financial statements submitted to the U.S. SEC (Securities and Exchange Commission). In particular, the plaintiff alleges that Niklason, Sander, and Prichard failed to exercise proper oversight and control over those financial statements.
For instance, on May 10, 2024, Humacyte reported its results for the first quarter of 2024 and stated that it was on track for the commercial launch of the Human Aceulllar Vessel (HAV), also called Acellular Tissue Engineered Vessel (ATEV), a lab-grown blood vessel that can replace injured or damaged blood vessels.
The company added that the Food and Drug Administration (FDA) accepted its Biologics License Application (BLA) for ATEV in the vascular trauma indication and that it was on track for another major milestone – the Prescription Drug User Fee Act (PDUFA) review on August 10, 2024.
However, subsequent disclosures revealed that the company allegedly misled investors about its prospects and failed to disclose some key risks.
Humacyte’s Misrepresentations
In contrast to the claims made by Humacyte and the other defendants, the company allegedly misrepresented the prospects of its ATEV offering. Notably, the company failed to disclose that its facilities were in poor condition and did not meet the FDA standards.
The truth started coming to light in August 2024. Specifically, on August 9, 2024, Humacyte announced that the FDA required additional time to complete the PDUFA review of its BLA for ATEV in the vascular trauma indication and that the regulatory body conducted inspections of its manufacturing facilities and clinical sites. Also, the company and FDA actively engaged in multiple discussions regarding its BLA filing, including post-marketing and labeling discussions.
Further, on October 17, 2024, the issues with Humacyte’s facilities became more clear when the FDA filed a Form 483, which revealed violations at the company’s North Carolina facility, including “no microbial quality assurance,” “no microbial testing,” and inadequate “quality oversight.”
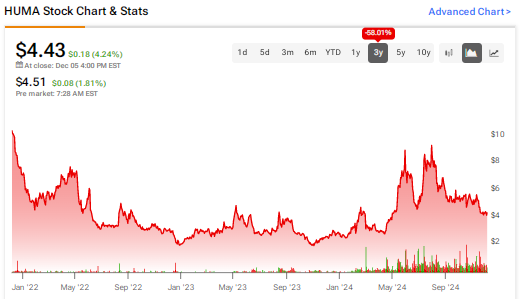
In reaction to the news, HUMA stock declined 16% in a single day.
To conclude, the defendants allegedly misled investors about the company’s prospects and failed to disclose the issues related to its manufacturing facilities. While HUMA stock has jumped 56% year-to-date, it has declined more than 58% over the past three years, leading to significant losses for shareholders.