Seres Therapeutics (MCRB), a microbiome therapeutics platform company, has reported Q1 earnings results that fell below Street expectations. First quarter GAAP EPS of -$0.28, missed by $0.02, while revenue of $8.19M (+11.9% Y/Y) missed by $0.35M.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
Seres ended 1Q20 with $75.1 mm in cash and equivalents, down from $94.8 mm at end 4Q19. This is sufficient to fund “operating expenses and capital expenditure requirements, excluding net cash flows from future business development activities or potential incoming milestone payments” into 2Q21, says the company.
On the impact of Covid-19, Seres announced “the timing of SER-287 Phase 2b and SER-401 Phase 1b clinical readouts is uncertain”, with no anticipated “disruptions to the availability of its drug product candidates for ongoing studies.”
Ser-287 is a microbiome therapy designed to improve the signs and symptoms associated with Ulcerative Colitis (UC), while Ser-401 aims to modify immunological activity and improve checkpoint inhibitor therapy responses.
Investors are also keeping a close eye on the phase III ECOSPOR III trial of Breakthrough Therapy Designated SER-109 in preventing recurrent C. difficile infection (rCDI).
“Even with Covid-19-related uncertainties on SER-287 and SER-401, we continue to see 2020 as a catalyst rich year for Seres on the ECOSPOR III results alone,” writes Chardan Capital analyst Gbola Amusa. His $12.50 price target indicates shares could more than double from the $4.59 current share price.
“Positive results could lead to SER-109 becoming the first FDA-approved microbiome medicine” says Amusa, noting that the current MCRB market cap is still modest in relation to the company’s pipeline.
He is now looking out for a webcast investor event focusing on the Ser-109 program on May 27 and top-line data from the phase III ECOSPOR III trial in rCDI in mid-2020. In addition Seres plans to initiate a 2020 patient dosing trial in Australia and New Zealand in ulcerative colitis (UC) and initiate a Ser-155 phase Ib trial in protection from, among other things, gastrointestinal infections.
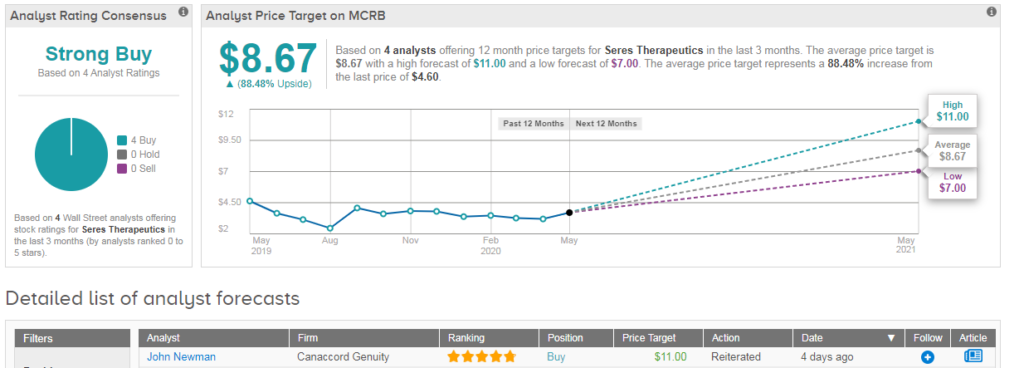
Overall the stock shows a bullish Strong Buy consensus from the Street, with all four analysts saying ‘buy.’ The $9 average analyst price target indicates significant upside potential of 88%, despite shares already rallying 33% year-to-date. (See Seres Therapeutics stock analysis on TipRanks).
Related News:
Pfizer Sets Wheels In Motion For Covid-19 Vaccine Production Ramp
AstraZeneca-Merck Ovarian Cancer Treatment Gets FDA Approval
Quidel’s Rapid Covid-19 Antigen Test Scores Emergency FDA Approval









