Shares of the medical research and drug development company, Sarepta Therapeutics (NASDAQ: SRPT) were down in morning trading on Thursday after a Stat News report indicated that certain FDA staff members were initially opposed to the company’s gene therapy candidate SRP-9001 before the FDA eventually agreed to hold an AdCom meeting on May 12 regarding its approval.
Maximize Your Portfolio with Data Driven Insights:
- Leverage the power of TipRanks' Smart Score, a data-driven tool to help you uncover top performing stocks and make informed investment decisions.
- Monitor your stock picks and compare them to top Wall Street Analysts' recommendations with Your Smart Portfolio
SRP-9001 is an investigational gene therapy treatment currently under the FDA review for Duchenne muscular dystrophy, a severe muscular dystrophy with a Prescription Drug User Fee Act (PDUFA) regulatory action date on May 29, 2023.
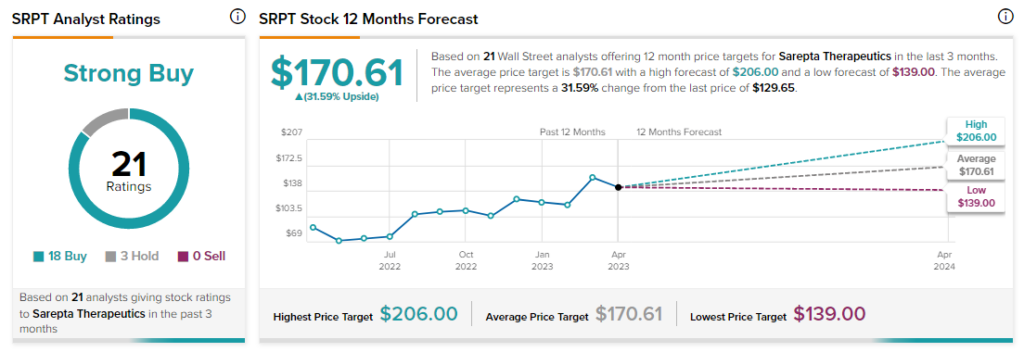
Analysts are bullish about SRPT stock with a Strong Buy consensus rating based on 18 Buys and three Holds.









