Sanofi’s CEO, Paul Hudson, said that the mRNA-based (messenger Ribonucleic acid) COVID-19 vaccine developed by the pharma giant and Translate Bio will not be ready this year, according to a Reuters report, citing the French newspaper Le Journal du Dimanche.
Invest with Confidence:
- Follow TipRanks' Top Wall Street Analysts to uncover their success rate and average return.
- Join thousands of data-driven investors – Build your Smart Portfolio for personalized insights.
Sanofi (SNYNF) expects to start the clinical trials of the mRNA-based vaccine this quarter, Reuters said, citing Le Journal du Dimanche. Hudson told the French newspaper, “This vaccine will not be ready this year, but it could be of use at a later stage all the more if the fight against variants was to continue.”
In October last year, the company had said that the pre-clinical trials of the experimental COVID-19 vaccine developed along with Translate Bio (TBIO) resulted in the production of neutralizing antibodies, indicating a favorable response from the immune system. At that time, the company said that it expected to begin a Phase 1 clinical trial in the last quarter of 2020.
The delay in the development of the vaccine could be another setback for Sanofi, in addition to the delay in the development of its COVID-19 vaccine along with Glaxo SmithKline (GSK). In December last year, Sanofi had said that a Phase 1 study of the antigen-based COVID-19 vaccine indicated that it was less effective in older adults as compared to adults in the age group of 18 to 49 years.
This prompted Sanofi to say that it plans to conduct a Phase 2b study in February this year with an improved antigen formulation of the vaccine, and expects to bring the vaccine to market in the fourth quarter of this year.
Sanofi has also collaborated with other companies to develop or manufacture a COVID-19 vaccine.
Late last month, Sanofi announced an agreement with BioNTech (BNTX), under which the company will provide BioNTech access to its production infrastructure for the COVID-19 vaccine. BioNTech is developing its COVID-19 vaccine along with Pfizer (PFE).
From the summer of this year, Sanofi will conduct late-stage manufacturing of the BioNTech vaccine and aims to supply 125 million doses to the European Union (EU) to increase the availability of the vaccine. (See Sanofi stock analysis on TipRanks)
Earlier this month, following the company’s 4Q and FY20 results, Liberum Capital analyst Alistair Campbell reiterated a Hold rating and a price target of $116.36 on the stock. Campbell said that the company’s 4Q and FY20 results were a “solid end” to the year, and while the analyst notes that the company’s outlook was slightly below consensus estimates, it appeared to be on the conservative side.
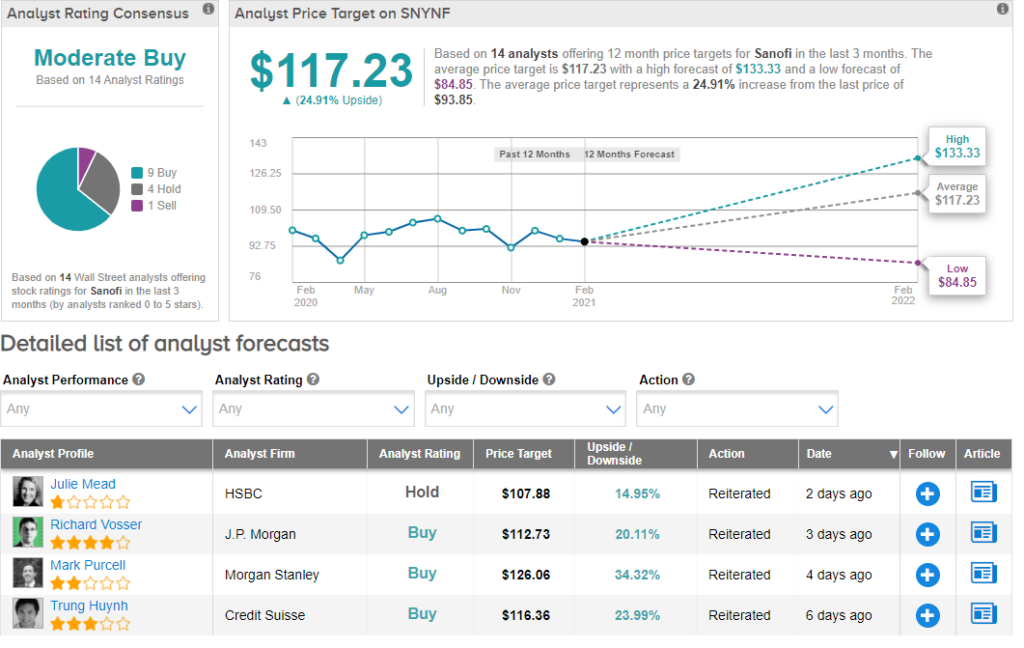
Meanwhile, Sanofi scores a Moderate Buy consensus rating from the analyst community. That’s based on 9 analysts recommending a Buy, 4 analysts suggesting a Hold and 1 analyst recommending a Sell. The average analyst price target of $117.23 implies 24.9% upside potential to current levels.
Related News:
J&J Files For FDA Approval Of COVID-19 Vaccine; Street is Bullish
Bio-Rad 4Q Sales Top Estimates Driven By COVID-19 Demand
Microsoft Showed Interest In Pinterest Takeover – Report









