Sanofi (SNYNF) and Regeneron have announced positive pivotal trial data for the investigational use of PD-1 inhibitor Libtayo (cemiplimab) in first-line locally advanced or metastatic non-small cell lung cancer (NSCLC) at the European Society for Medical Oncology (ESMO) Virtual Congress 2020.
The trial compared Libtayo monotherapy to platinum-doublet chemotherapy in patients whose tumor cells expressed PD-L1, including those whose cancers had confirmed PD-L1 expression of ≥50%.
Lung cancer is the leading cause of cancer death worldwide, with more than 2.2 million new cases expected globally in 2020. Approximately 85% of all lung cancers are NSCLC, and an estimated 25% to 30% of these cases are expected to test positive for PD-L1 in ≥50% of tumor cells.
“In new analyses presented at ESMO, Libtayo reduced the risk of death by 43% in patients whose cancer had confirmed PD-L1 expression of 50% or greater. This is notable given that nearly three-quarters of patients crossed over from chemotherapy following disease progression and 12% of patients had pretreated and stable brain metastases,” said Ahmet Sezer, M.D., a trial investigator.
“These results support Libtayo as a potential new option for anti-PD-1 monotherapy in first-line advanced non-small cell lung cancer” he added.
In the overall trial population (n=710), the median follow-up was 13 months for both Libtayo and chemotherapy. Among these patients, Libtayo demonstrated the following results compared to chemotherapy: 32% reduced risk of death; 22-month median overall survival compared to 14 months; 41% reduced risk of disease progression; median progression-free survival (PFS) of 6.2 months compared to 5.6 months; and a 37% objective response rate (ORR) compared to 21%.
A prespecified analysis of data from patients whose cancers had confirmed PD-L1 expression ≥50% (n=563) was also conducted. Here Libtayo demonstrated the following results vs chemotherapy: 43% reduced risk of death; 46% reduced risk of disease progression; the median PFS was 8 months vs 6 months; and 39% ORR vs 20% ORR.
The trial also found a direct correlation between tumor response and PD-L1 expression level in Libtayo-treated patients. The ORR was highest (46%; range: 36-56%) in tumors with ≥90% PD-L1 expression, with target tumors shrinking by more than 40% after 6 months of treatment on average (per last observation carried forward method). This correlation with PD-L1 expression level was not observed with chemotherapy.
Overall adverse events (AEs) occurred in 88% of Libtayo patients and 94% of chemotherapy patients. Grade 3 or higher AEs occurred in 37% of Libtayo patients and 49% of chemotherapy patients. No new Libtayo safety signals were observed.
Libtayo is a fully-human monoclonal antibody targeting the immune checkpoint receptor PD-1 on T-cells. By binding to PD-1, Libtayo has been shown to block cancer cells from using the PD-1 pathway to suppress T-cell activation.
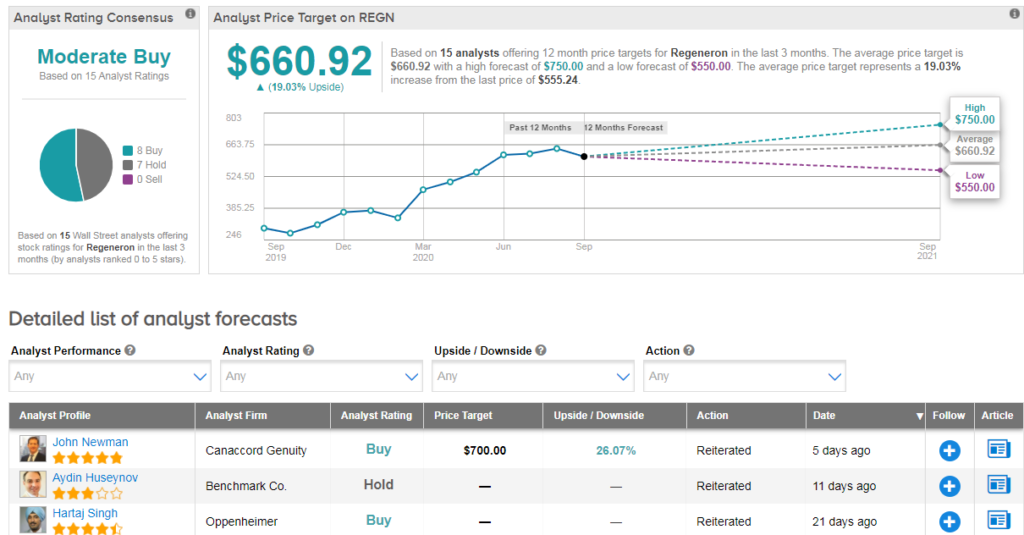
Turning to the Street, Sanofi shows a bullish Strong Buy Street consensus, while Regeneron (REGN) scores a slightly more cautious Moderate Buy consensus. Meanwhile the average analyst price target indicates roughly 20% upside potential lies ahead for both stocks. However Sanofi has only climbed 4.5% year-to-date, while Regeneron has exploded 48%.
“REGN is on the cusp of: (1) strong 20/21 sales growth, (2) increased Eylea penetration in DME in 2020/21; (3) approval, launch, and fast uptake of Dupixent in asthma/other allergic conditions; and (4) approval, launch, and fast uptake of Libtayo in advanced cSCC/other oncology indications” commented Oppenheimer’s Hartaj Singh recently.
He has a buy rating on the stock, adding “we continue to believe investors should own the three leaders (respectively) in the COVID-19 race; GILD, REGN, and MRNA.” (See REGN stock analysis on TipRanks).
Related News:
Eli Lilly’s Verzenio Treatment Lowers Breast Cancer Recurrence By 25%
Novavax Inks Deal With India For 2B Covid-19 Vaccine Doses In 2021
Merck Puts Focus On Lower Debt, Sees Smaller Takeovers After 2022
Questions or Comments about the article? Write to editor@tipranks.com
















