Drugmaker Sanofi and its British partner GlaxoSmithKline delayed the availability of their adjuvanted recombinant protein-based COVID-19 vaccine program to the end of 2021, after trial data results showed an insufficient immune response in elderly patients.
Specifically, Sanofi (SNYNF) reported that the vaccine candidate’s Phase 1/2 study interim results showed an immune response comparable to patients who recovered from COVID-19 in adults aged 18 to 49 years, but a low immune response in older adults likely due to an insufficient concentration of the antigen. The two companies added that the insufficient response in older adults now requires a reformulation of the concentration of antigen in order to achieve a high-level immune response across all age groups.
As a result, Sanofi and GSK (GSK) are now planning to initiate a Phase 2b study with an improved antigen formulation. The study is expected to start in February 2021 and will include comparison with an authorized COVID-19 vaccine.
“The results of the study are not as we hoped. Based on previous experience and other collaborations, we are confident that GSK’s pandemic adjuvant system, when coupled with a COVID-19 antigen, can elicit a robust immune response with an acceptable reactogenicity profile,” commented GSK’s Roger Connor. “It is also clear that multiple vaccines will be needed to contain the pandemic. Our aim now is to work closely with our partner Sanofi to develop this vaccine, with an improved antigen formulation, for it to make a meaningful contribution to preventing COVID-19.”
If the Phase 2b study data are positive, a global Phase 3 study could start in Q2 2021, the two companies said. Furthermore, positive results from the study would now target regulatory submissions in the second half of 2021. Hence, the companies delayed the vaccine’s potential availability from mid-2021 to Q4 2021.
The Sanofi-GSK protein-based vaccine candidate was selected in July 2020 by the US government’s Operation Warp Speed in order to support and accelerate its development and manufacturing.
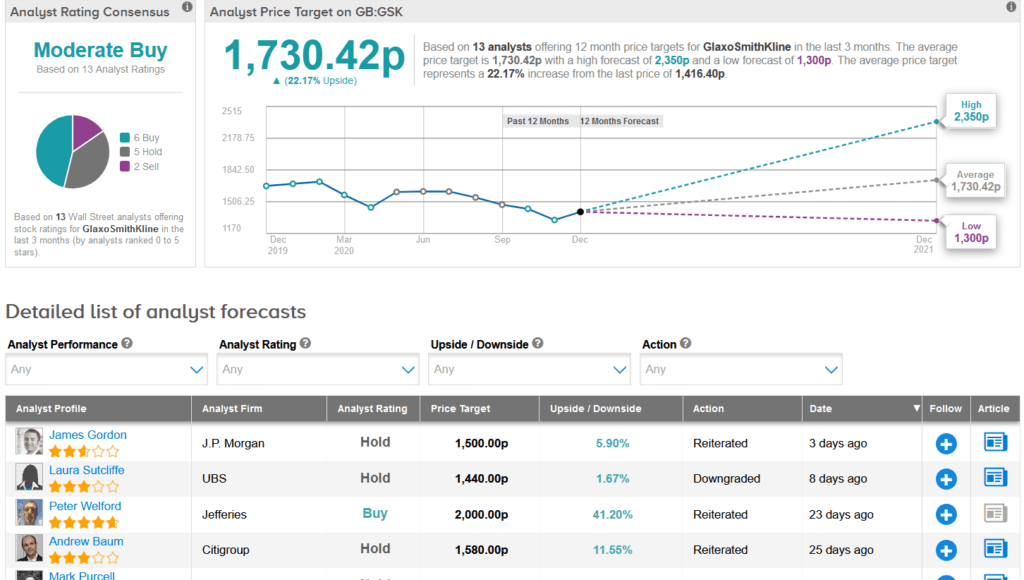
Earlier this month, UBS analyst Laura Sutcliffe cut GSK’s rating to Hold from Buy with a 1,440p price target (1.7% upside potential), as she views the European pharma sector as “cheap but not a bargain.”
Commenting specifically on GSK, Sutcliffe believes that investors will move toward a sum-of-the-parts valuation until things are clearer on the separation of its consumer health joint venture with Pfizer. (See GSK stock analysis on TipRanks)
The rest of the Street is cautiously optimistic on the stock with a Moderate Buy analyst consensus. That’s based on 6 Buys versus 5 Holds and 2 Sells. With shares down 20% so far this year, the average analyst price target stands at 1,730.42p, implying 22% upside potential lies ahead in coming months.
Related News:
Pfizer-BioNTech’s Covid-19 Vaccine Wins FDA Panel Vote; Shares Rise
Immutep Explodes 63% On Encouraging Breast Cancer Trial Data
Pfizer-BioNTech Covid-19 Vaccine Gets FDA Safety Vote; Mizuho Says Buy
















