The at-home COVID-19 rapid test developed by Swiss multinational healthcare company Roche Holdings (RHHVF) received Emergency Use Authorization (EUA) by the U.S. Food and Drug Administration.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
Roche delivers personalized healthcare solutions backed by combined strengths of pharmaceuticals and diagnostics, along with growing capabilities in data-driven medical insights.
At-Home COVID-19 Rapid Test
The product will help people to get reliable results within 20 minutes for all known variants of concern, including Omicron, by collecting a simple anterior nasal swab sample. Notably, Roche revealed that the product will be available across the United States as early as January.
Roche is launching the product in partnership with SD Biosensor Inc., with whom it has a global distribution agreement. The FDA has prioritized at-home testing due to Roche and SD Biosensor’s capacity to provide large quantities of tests and the ability to ramp up manufacturing to meet future demands.
Along with the rapid test solution, Roche is offering NAVIFY® Pass, that will allow individuals and health care professionals to remotely and securely store, display, and share results.
President & CEO of Roche Diagnostics North America, Matt Sause, said, “As long as there remains a need for reliable testing, Roche will continue to invest in effective solutions to ensure there are testing options available to those who need them.”
Amid the rising COVID-19 cases, other Biotech stocks are making efforts to bag approvals for at-home treatments. Last week, Pfizer’s (PFE) COVID-19 oral antiviral treatment, PAXLOVID, received authorization for use from the FDA for people aged 12 and older and weighing at least 40 kg.
Wall Street’s Take
The rest of the Street is cautiously optimistic about the stock, with a Moderate Buy consensus rating based on 6 Buys and 7 Holds. The Roche price target of $421.81 implies 2.89% upside potential to current levels.
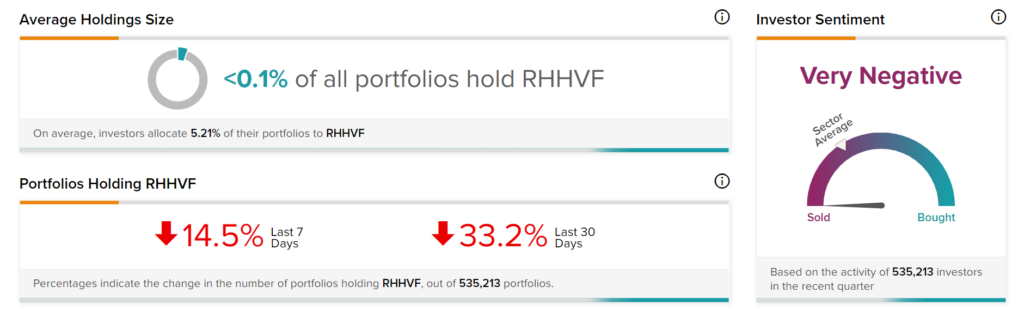
Negative Investor Sentiments
TipRanks’ Stock Investors tool shows that investors currently have a Very Negative stance on Roche, with 33.2% of investors decreasing their exposure to RHHVF stock over the past 30 days.
Related News:
Freshworks Reveals Settlement of Lawsuit Filed by Zoho
Merck & Ridgeback’s Molnupiravir Receives Special Emergency Approval in Japan
Cassava Introduces Clinical Website to Aid Phase 3 Simufilam Study









