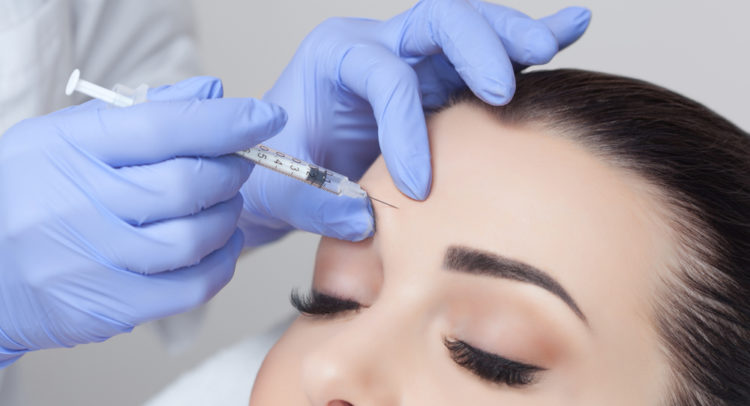
Shares of neuromodulators developer Revance Therapeutics (NASDAQ:RVNC) continue to fly higher today after the biotechnology company’s peptide-formulated neuromodulator – DAXXIFY won approval from the U.S. Food and Drug Administration (FDA).
Maximize Your Portfolio with Data Driven Insights:
- Leverage the power of TipRanks' Smart Score, a data-driven tool to help you uncover top performing stocks and make informed investment decisions.
- Monitor your stock picks and compare them to top Wall Street Analysts' recommendations with Your Smart Portfolio
DAZZZIFY (DaxibotulinumtoxinA-lanm) for injection has received approval for temporary improvement of frown lines for adults. This is the first FDA approval for Revance and enables the company to tap the $3.2 billion U.S. facial injectables market.
The approval came on data from the Phase 3 SAKURA study (over 2,700 patients) and the product offers year-round results with as many as only two treatments a year.
The CEO of Revance, Mark J. Foley noted, “We are very pleased DAXXIFY’s label includes data demonstrating the achievement of none or mild wrinkle severity based on investigator and subject assessments, as this provides the foundation for our marketing claims around the duration of effect.”
The company’s current pipeline is targeting muscle movement disorders.
Is RVNC Good for the Long-Term?
While Revance currently scores a Strong Buy consensus rating from Wall Street, the average price target of $25 indicates shares may be fairly priced at current levels already.

Read full Disclosure