Regeneron Pharmaceuticals revealed encouraging initial data from its ongoing Phase 1/2/3 clinical trial of its antibody cocktail (casirivimab and imdevimab) in hospitalized COVID-19 patients on low-flow oxygen.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
The analysis focuses on patients who had not yet mounted their own immune response to SARS-CoV-2 (which means these patients did not have antibodies at baseline, or seronegative). Regeneron (REGN) explained that the primary clinical objective of this initial analysis was to determine if there was sufficient efficacy in these patients for continuing the trial (futility analysis).
“The results passed the futility analysis (p<0.3 one-sided), as seronegative patients treated with the antibody cocktail had a lower risk of death or receiving mechanical ventilation (HR: 0.78; 80% CI: 0.51-1.2). The benefit was driven by results starting one week post-treatment, when the risk of death or receiving mechanical ventilation was reduced by approximately half with antibody cocktail treatment, based on a post-hoc analysis,” said the company in a statement.
Meanwhile, George D. Yancopoulos, Regeneron’s President and Chief Scientific Officer cautioned that while the virology results from this analysis of hospitalized patients were robust, the clinical efficacy data is based on a small data set of events and cannot be viewed as “conclusive” currently. (See REGN stock analysis on TipRanks)
Yancopoulos stated, “A much larger trial will be required to rigorously characterize this effect and we believe the ongoing UK-based RECOVERY trial will provide those answers. It has already enrolled more than 2,000 hospitalized patients in the part of the trial evaluating adding the antibody cocktail to standard-of-care compared to standard-of-care alone.”
Last month, Regeneron’s antibody cocktail was granted an Emergency Use Authorization (EUA) by the US FDA in high-risk patients who have confirmed COVID-19 but are not currently hospitalized.
Following the FDA’s EUA, Piper Sandler analyst Christopher Raymond reiterated a Buy rating with a price target of $675 on Regeneron. While Raymond admitted that the news came a little later than he expected, he is nonetheless encouraged that the prior guidance of shipping the 300,000-dose BARDA order by the end of January remains.
Raymond believes the antibody cocktail could be a meaningful earnings contributor and add $4 per share or more to the company’s 2021 earnings. He continues to feel that this can be a “durable” $1 billion plus business.
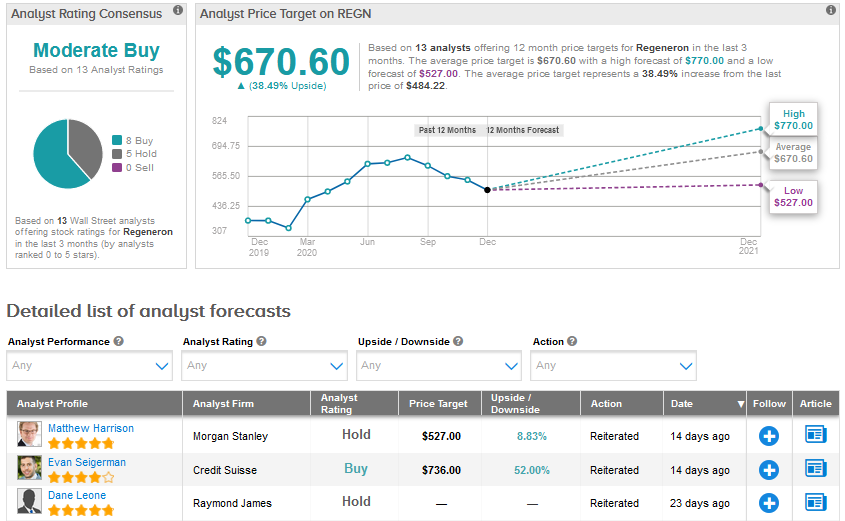
Overall, Regeneron scores a Moderate Buy analyst consensus based on 8 Buys and 5 Holds. The average price target stands at $670.60, indicating an upside potential of 38.5% in the months ahead. Shares have already risen 29% year-to-date.
Related News:
Pfizer, BioNTech to Deliver Additional 100M Vaccine Doses to EU
Quanterix Wins FDA Approval For Covid-19 Antibody Test; Shares Rise 6%
Arcturus Gets Approval For Covid-19 Vaccine Phase 2 Study; Shares Plunge 38%









