RedHill Biopharma Ltd. said that preliminary data results from the US Phase 2 study of its opaganib administered in patients hospitalized with COVID-19 pneumonia demonstrated positive safety and efficacy signals. Meanwhile, shares closed 5.6% lower on Dec. 31.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
RedHill Biopharma (RDHL) reported that the findings of the Phase 2 study of 40 hospitalized patients requiring oxygen support demonstrated that opaganib was safe, with no material safety differences between opaganib and placebo treatments. The opaganib-treated group showed greater improvement in reducing oxygen requirement by end of treatment at day 14 across key primary and secondary efficacy outcomes. The focus of the study was to evaluate safety and identify preliminary signs of activity, rather than statistical differences, the company said.
Opaganib is a first-in-class, orally-administered, sphingosine kinase-2 (SK2) selective inhibitor with demonstrated dual anti-inflammatory and antiviral activity. It targets a human cell component involved in viral replication, potentially minimizing the likelihood for resistance due to viral mutations.
“We are pleased with these encouraging top-line results from our exploratory Phase 2 study which confirm opaganib’s safety and demonstrate promising signals of activity when treating patients with COVID-19 and who require oxygen support,” commented RedHill Medical Director Mark L. Levitt. “We continue to work diligently to compile a robust data set to support potential filing of global emergency use applications.”
The efficacy of opaganib as a treatment for patient with severe COVID-19 pneumonia is being further explored in an ongoing global Phase 2/3 study. The study is being conducted across 30 clinical sites in several countries and is on track to enroll up to 270 patients. RedHill expects to release top-line data results from the study in the first quarter of 2021.
Shares in RedHill Biopharma have dropped 6.3% over the past month, trimming their 2020 gain to 33%.
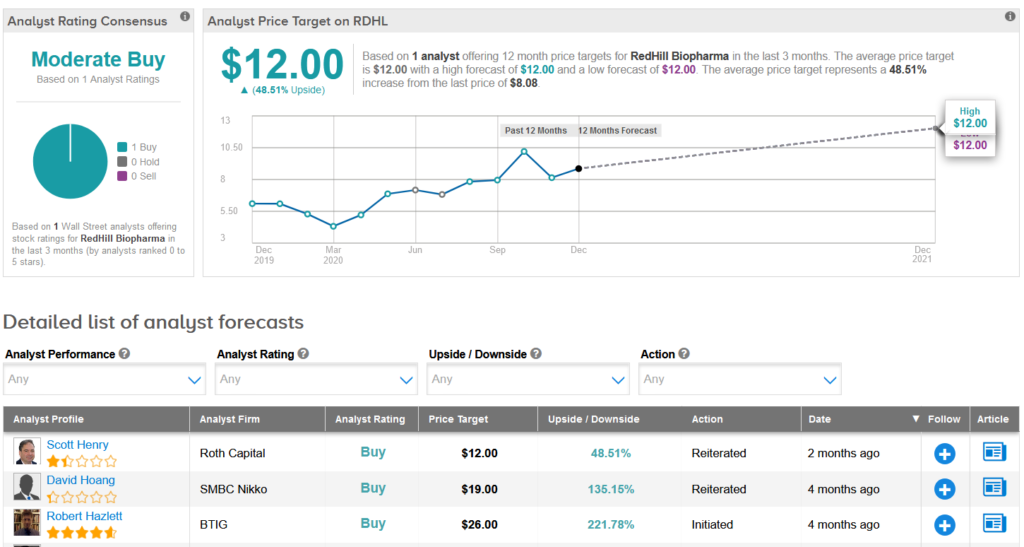
The stock was most recently reviewed by Roth Capital analyst Scott Henry, who cut the price target to $12 (49% upside potential) from $16 following the company’s Q3 results, which were impacted by the COVID-19 crisis.
However, Henry maintained a Buy rating on RDHL, calling the company’s COVID-19 therapeutic program “a wildcard”, with data likely expected in Q1 of 2021. (See RDHL stock analysis on TipRanks)
Related News:
Moderna In Talks To Supply South Korea With 40M Covid-19 Vaccine Doses; Shares Gain
Patterson Companies Falls 5% On Loss of Heartland Dental Distribution Deal
Incyte Partners With Cellenkos For Blood Cancer Therapy; Street Sees 13% Upside









