Recursion Pharmaceuticals (RXRX) is a clinical-stage biotechnology company utilizing artificial intelligence to expedite drug discovery and development. With its stock taking a hit due to the lack of validation of its AI-powered platform, anticipation is building for the results of a phase 2 trial with REC-2282, which could boost Recursion shares if successful. Recently, Recursion released promising data from a phase 2 study of another drug, REC-994, to treat Cerebral Cavernous Malformations (CCM). Preliminary results indicate the safety of REC-994 and a reduction in lesions among 50% of patients following a year of treatment.
These findings carry significant potential, and the company looks forward to collaborating with the FDA and the CCM community to take its next steps. The stock presents a high-risk, high-potential-reward opportunity for investors with iron-clad stomachs.
Recursion’s Unique Approach
Recursion Pharmaceuticals is a clinical-stage “TechBio” company at the forefront of leveraging advances in artificial intelligence (AI) for drug discovery. The company’s efforts are enabled by the Recursion OS, a platform that integrates diverse technologies to continuously grow a proprietary biological and chemical dataset, one of the largest in the world.
Recursion harnesses robust machine-learning algorithms to extract trillions of searchable relationships from its dataset, circumventing human bias. Its approach also involves commanding significant experimental and computational scale, including conducting millions of wet lab experiments every week.
Investments, Collaborations, and Trials Advancements
The firm has secured a significant investment from NVIDIA (NVDA). It has further strengthened its collaboration with Google Cloud (GOOG) to enhance its drug discovery platform and advance its exploration of generative AI capabilities. Further, the company boasts ongoing partnerships with global pharmaceutical firms Bayer (BYRY) and Roche (RHHBY) in developing new treatments.
The company announced the completion of its Phase 2 SYCAMORE clinical trial of REC-994, an innovative treatment targeted at Cerebral Cavernous Malformations (CCM). Furthermore, REC-994 has shown promising efficacy, with 50% of patients experiencing a reduction in mean lesion volume after 12 months of treatment, particularly at the higher 400 mg dose.
REC-994 has proven to be effective, particularly in patients with brainstem lesions whom surgery is not an option for. This offers an exciting new alternative for high-need patients. Recursion intends to continue this investigation in partnership with regulatory discussions and forward-looking extension studies.
Recent Financial Results Missed on The Top-and-Bottom-line
Recursion recently revealed its Q3 financials, reporting a lower-than-expected revenue of $26.1 million, a considerable increase from the previous year’s Q3 revenue of $10.5 million. This increase is attributed to a partnership with Roche & Genentech and a $30.0 million acceptance fee for completing a neuroscience project. However, the revenue missed expectations by $3.73 million.
Operational expenses also surged, with research and development expenses standing at $74.6 million and general and administrative costs amounting to $37.8 million. This rise in expenses primarily resulted from enhancements and expansions to the company’s platform, such as its chemical technology, machine learning, and transcriptomics platform, as well as increased software and lease expenses.
The net loss marginally increased from $93.0 million in Q3 2023 to $95.8 million in Q3 2024, with a per-share loss of -$0.34, missing the consensus estimates by $0.01.
At the quarter’s end, cash and cash equivalents totaled $427.6 million. Net cash utilized in operating activities fell from $72.9 million in Q3 2023 to $59.2 million in Q3 2024, mainly due to the receipt of the acceptance fee that partly countered the increased operational costs.
Cautious Optimism on the RXRX Stock
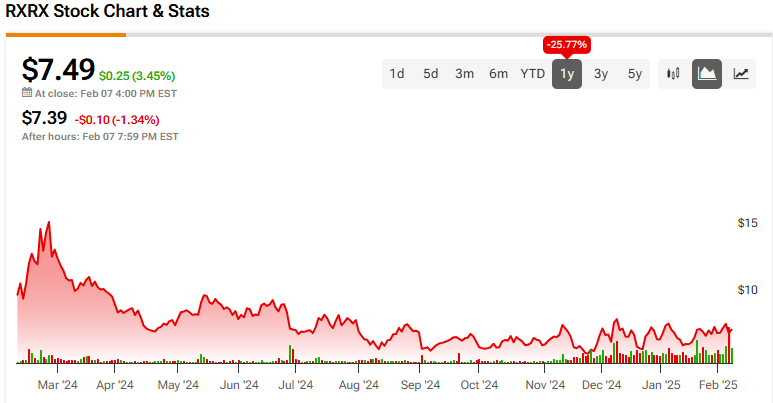
The stock has been highly volatile, bouncing around between $5.60 and $15.74 in the past year. It currently trades at the lower end of that range, yet a recent run has the shares showing positive price momentum as they trade above the major moving averages.
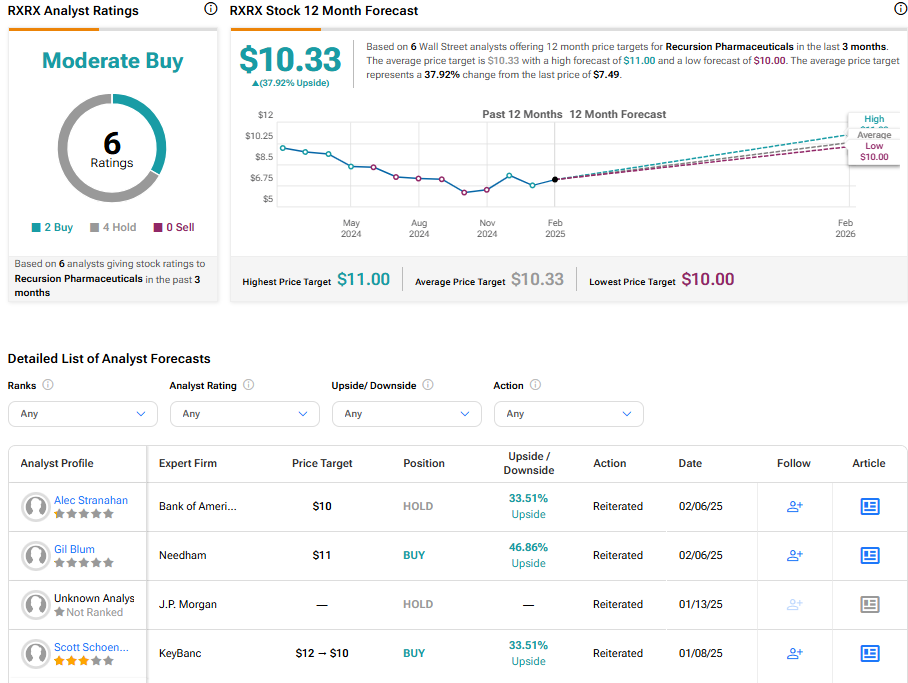
Analysts following the company have been cautiously optimistic about RXRX stock. For instance, JP Morgan (JPM) has recently reiterated a Neutral rating, noting it will take definitive clinical wins from Recursion’s pipeline to validate the platform.
Based on the recent recommendations of six analysts, Recursion Pharmaceuticals is rated a moderate buy overall. The Average price target for RXRX stock is $10.33, representing a potential upside of 37.92% from current levels.
Recursion Pharmaceuticals in Review
Recursion Pharmaceuticals has shown resilience in the face of recent setbacks, showing promising results in recent drug trials. Particularly, results from the Phase 2 SYCAMORE trial of REC-994, a potential new treatment for Cerebral Cavernous Malformations, are promising. The stock has been volatile but is currently showing positive momentum. For investors with a risk appetite, Recursion presents an intriguing opportunity in a pioneering field.
Questions or Comments about the article? Write to editor@tipranks.com
















