Clinical-stage, immunology-based biopharmaceutical company RAPT Therapeutics (NASDAQ:RAPT) crashed over 60% in today’s trading. This comes after it announced that the U.S. Food and Drug Administration (FDA) placed a clinical hold on the company’s Phase 2b trial of zelnecirnon (RPT193) for atopic dermatitis (dry, itchy, and inflamed skin) and its Phase 2a trial for asthma.
Don't Miss our Black Friday Offers:
- Unlock your investing potential with TipRanks Premium - Now At 40% OFF!
- Make smarter investments with weekly expert stock picks from the Smart Investor Newsletter
The FDA placed this clinical hold after a patient suffered acute liver failure in the atopic dermatitis trial. No new participants will be enrolled in the trials. The clinical hold, however, excludes the tivumecirnon trial for cancer.
RAPT is thoroughly investigating this case, which involved a patient with a complex medical history who reportedly had a COVID-19 infection during the time of the event.
Is RAPT a Good Stock to Buy?
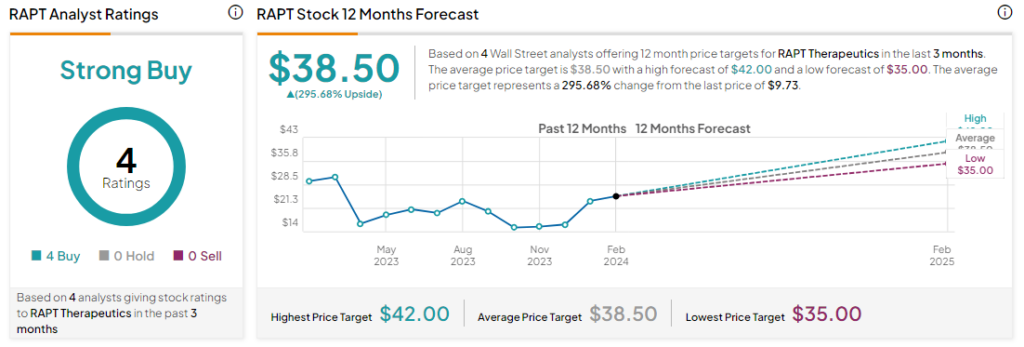
Analysts remain bullish about RAPT with a Strong Buy consensus rating based on four Buys. RAPT has plunged by more than 69% over the past 12 months, and the average RAPT price target of $38.50 implies an upside potential of more than 200% at current levels. However, it’s worth noting that estimates will likely change following today’s announcement.