Shares of Quanterix Corp. spiked almost 10% in Monday’s extended trading session after the Food and Drug Administration (FDA) authorized emergency use for its SARS-CoV-2 N protein antigen test.
Quanterix’s (QTRX) antigen test detects the presence of the SARS-CoV-2 virus nucleocapsid protein, which is elevated in respiratory fluids during the initial acute phase of the infection. The company argues that direct detection of antigen proteins from the virus may be a more “meaningful” measure of infection status than detection of RNA by rRT-PCR because genetic material can linger even after the virus has left the body, which in turn increases the risk of false positives.
In clinical studies, the test showed sensitivity of 97.7% and specificity of 100% up to 14 days following the onset of symptoms.
Specifically, the FDA cleared the antigen test for use with nasopharyngeal (NP) samples in individuals suspected of being infected with COVID-19 by their healthcare providers.
“As case counts and positivity rates rise globally, the COVID-19 testing landscape is of critical importance,” said Quanterix CEO Kevin Hrusovsky. “Antigen viral levels, if precisely tested, are potentially a better measure of active infection, and for understanding the kinetics and distribution of viral load across different sample types over the duration of the infection, treatment and recovery. We are pleased to announce a test that not only provides high levels of sensitivity and specificity, but is economical, well suited for high throughput testing and utilizes a complementary supply chain helping efforts to scale testing.”
According to preliminary clinical research studies, the viral antigen may be readily detectable in asymptomatic and pre-symptomatic patients and therefore, Quanterix is exploring extending the test to screening applications, home-based sample collection and pooling to enable larger scale testing.
Going forward, Quanterix said that the company will also be seeking authorization for additional sample types, including nasal swabs, saliva, and capillary dried blood obtained from a fingerstick.
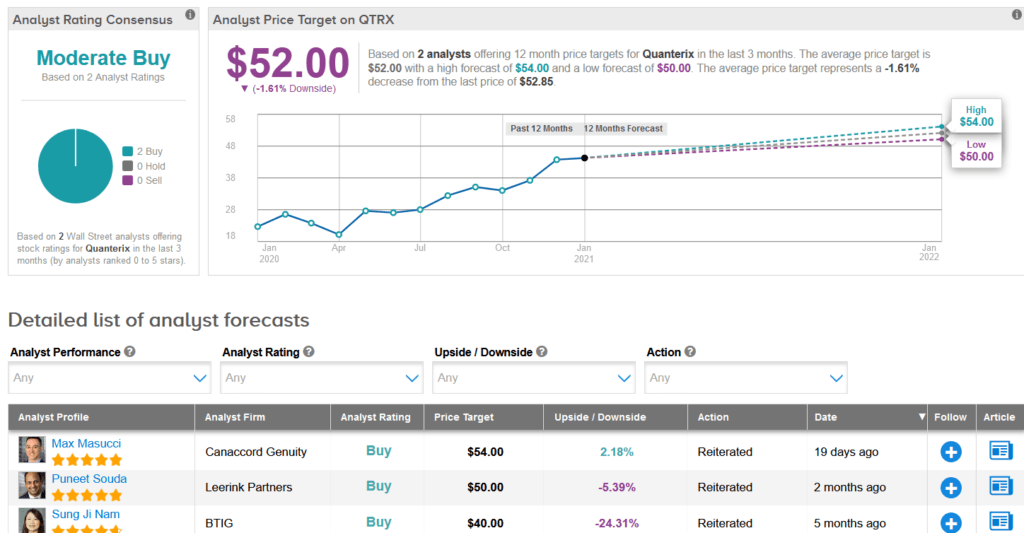
Canaccord Genuity analyst Max Masucci last month reiterated a Buy rating on the stock with a price target of $54 (2.2% upside potential) as he expects QTRX to be a “multi-year winner in the research and clinical markets.”
“We suspect that QTRX is making solid progress with its development of a high-sensitivity antigen test to accurately diagnose COVID-19 in asymptomatic patients (a major limitation that plagues commercially available COVID-19 tests),” Masucci wrote in a note to investors. (See QTRX stock analysis on TipRanks)
Overall, the rest of the Street has a cautiously optimistic outlook on the stock. The Moderate Buy analyst consensus is based on 2 unanimous Buys. With shares up a whopping 144% over the past year, the average price target stands at $52 and implies 1.6% downside potential at current levels.
Related News:
EasyJet Signs New Five-Year $1.87B Loan; Shares Drop
Sanofi To Snap Up Kymab For Up To $1.45B; Street Sees 25% Upside
Carnival’s Aida Cruise Line Extends Sailings Halt; Street Sticks To Hold