Pharmaceutical giant Pfizer, Inc. (PFE) and Germany-based biotechnology company BioNTech SE (BNTX) have submitted data to the U.S. Food and Drug Administration (FDA) for emergency use authorization of its COVID-19 vaccine for children aged 5 – 11 years, as per a Reuters report.
Shares of PFE rose 1.7%, closing at $42.74, and BNTX popped 4.4%, closing at $246.57 on October 7.
So far, there is no vaccine shot available for this age group, and with the surge of the Delta variant and COVID-19 cases, this approval is a step forward in protecting children and the overall population against the deadly virus. On September 20, the companies provided data showing a strong immune response to the vaccine in children aged 5 – 11, conducted with a 2,268-participant clinical trial. (See Pfizer stock charts on TipRanks)
The FDA has assigned a panel of outside advisors to discuss the application and has until October 26 to finalize its view, the report said. There are about 28 million children in the 5 – 11 age group in the U.S. who will receive the vaccine if approved.
As per the American Academy of Pediatrics, children account for about 27% of all U.S. coronavirus cases, with an increasing rate of hospitalizations. Though kids do not get severely affected by the COVID-19 virus, they can be carriers of the virus and make the population vulnerable, especially those who are unvaccinated and those with pre-existing medical conditions.
If the vaccine is approved, the two-dose Pfizer/BioNTech vaccine will be the first for the 5 – 11 age group and would help in curbing coronavirus cases going forward, with schools open nationwide and winter approaching.
The Pfizer/BioNTech vaccine is one of the three COVID-19 vaccines in the U.S. with emergency use authorization for teens aged 12 – 15 and full approval for ages 16 and above. While the other two vaccines, the two-dose Moderna shot and the single-dose Johnson & Johnson shot, have not got full approval for all age groups.
The two companies are also expected to submit vaccine test results to the FDA in the fourth quarter for children aged 2 – 5 years, as well as children aged 6 months to 2 years.
Recently, Mizuho Securities analyst Vamil Divan reiterated a Hold rating on the stock with a price target of $43, implying that shares are almost fully valued at current levels.
Divan noted that Pfizer’s shares have been volatile lately due to COVID-19 vaccine-related news.
After meeting with Pfizer’s Investor Relations (IR) team, Divan said, “We are encouraged by the success the company has had with the vaccine and expect the significant cash flows Pfizer is now receiving to provide the company with significant optionality on the business development front. However, we wait to see how management actually allocates its capital and how it improves Pfizer’s 2026-2030 outlook before becoming more constructive on the stock.”
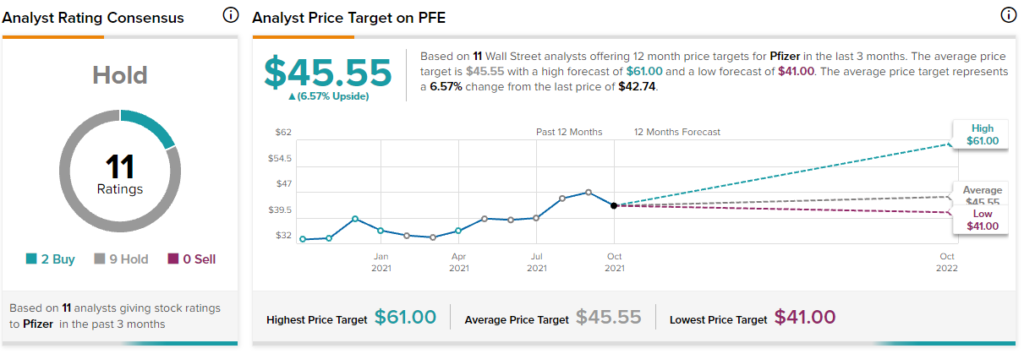
Overall, the stock has a Hold consensus rating based on 2 Buys and 9 Holds. The average Pfizer price target of $45.55 implies 6.6% upside potential to current levels. Shares have gained 22.3% over the past year.
Related News:
Tesla Hikes Prices of Model 3 and Model Y
Levi’s Beats Q3 Expectations; Shares Pop After-Hours
Paychex Acquires Flock to Drive Innovation
















