Pfizer Inc. (NYSE: PFE) has disclosed positive top-line results from the Phase 3 study (B7471026) of PREVNAR 20 (Pneumococcal 20-valent Conjugate Vaccine) when administered together with the Pfizer-BioNTech COVID-19 vaccine. The study included 570 adults in the United States aged 65 or older.
The trial was designed to evaluate the safety and immunogenicity of PREVNAR 20 when administered along with the Pfizer-BioNTech COVID-19 vaccine, or with a placebo.
Notably, PREVNAR 20 is Pfizer’s next-generation pneumococcal conjugate vaccine consisting of capsular polysaccharide conjugates for the 13 serotypes. In addition, the vaccine contains capsular polysaccharide conjugates for seven additional serotypes that have been associated with high fatality rates.
The study was initiated in May 2021 to explore the administration of PREVNAR 20 along with the booster dose of the Pfizer-BioNTech COVID-19 vaccine in older adults. Adults from the pivotal Phase 3 Pfizer-BioNTech COVID-19 vaccine clinical trial and adults who were administered the second dose of the vaccine a minimum of six months before the study participated in the trial. Detailed results are expected to be announced at a future date.
Official Comments
SVP and Head of Vaccine Research & Development at Pfizer, Kathrin U. Jansen, said, “Pfizer is steadfast in its commitment to address the burden of certain respiratory diseases while raising awareness of the importance of adult immunizations. These new safety and immunogenicity data provide further evidence supporting the potential to administer PREVNAR 20 and the Pfizer-BioNTech COVID-19 Vaccine at the same time, thereby reducing the number of visits adults make to their doctor’s office or pharmacy for recommended immunization.”
“As the COVID-19 vaccines and booster doses continue to be administered, we believe that healthcare providers have an opportunity to talk to their adult patients about other recommended vaccines in line with CDC guidance,” Jansen added.
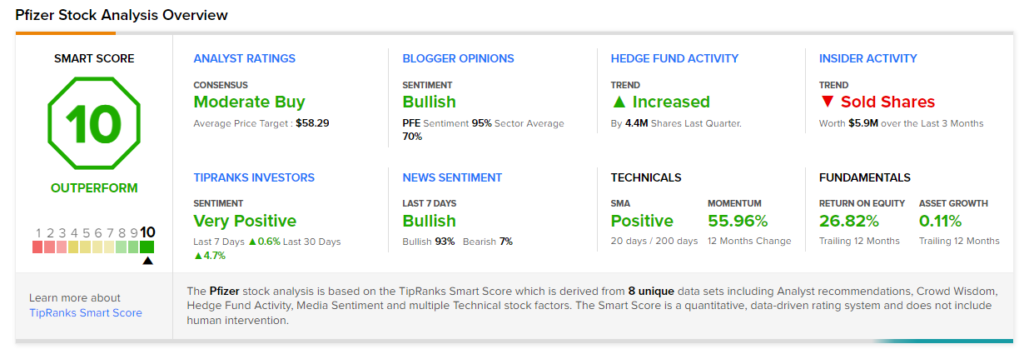
Wall Street’s Take
Recently, Barclays analyst Carter Gould maintained a Hold rating and a price target of $54 (4.68% downside potential) on the stock.
The rest of the Street is cautiously optimistic about the stock, with a Moderate Buy consensus rating based on 8 Buys and 10 Holds. The average Pfizer price target of $58.29 implies 2.89% upside potential to current levels. Shares have gained 60.2% over the past year.
Smart Score
Pfizer scores a “Perfect 10” from TipRanks’ Smart Score rating system. This makes it one of TipRanks’ Top Stocks and implies that the stock has strong potential to outperform market expectations.
Download the TipRanks mobile app now
To find good ideas for stocks trading at attractive valuations, visit TipRanks’ Best Stocks to Buy, a newly launched tool that unites all of TipRanks’ equity insights.
Read full Disclaimer & Disclosure
Related News:
Hackers Retweet Elon Musk after Hacking Ministry of Information and Broadcasting
U.S. Government to Purchase 600,000 Additional Doses of Sotrovimab; Shares Jump
CVS Health Expects Higher Earnings in 2021
Questions or Comments about the article? Write to editor@tipranks.com
















