Pfizer and German partner BioNTech announced that the European Commission (EC) has granted a conditional marketing authorization (CMA) to use its BNT162b2 vaccine for immunization to prevent COVID-19 in individuals aged 16 years and older.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
Pfizer (PFE) said that the approval was based on the positive opinion by the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP), to authorize the COVID-19 vaccine. The CMA is valid in all 27 member states of the European Union (EU). BNT162b2 is the first COVID-19 vaccine to be granted CMA in the EU. The decision comes after the UK and the US approved the vaccine’s emergency use in early December.
Following the approval, Pfizer and BioNTech will start the delivery of the first vaccine doses immediately across the EU. In November, the two companies reached an agreement to supply 200 million vaccine doses in 2020 and 2021, with the option for up to 100 million additional doses.
“The conditional marketing authorization by the European Commission is an historic achievement,” said BioNTech CEO, Ugur Sahin. “As a company founded and headquartered in the heart of Europe, we are looking forward to delivering the vaccine to Europeans in the upcoming days. We believe that vaccinations may help reduce the number of people in high-risk populations being hospitalized.”
Sahin added that going forward, the companies are working on collating efficacy and safety data in trial participants over the coming two years and will be testing the vaccine against additional mutations.
The EU authorization is based on scientific data, including from a pivotal Phase 3 clinical study released last month, which demonstrated a vaccine efficacy rate of 95% in participants without prior SARS-CoV-2 infection.
Shares of Pfizer have declined 4.7% over the past five days and are up about 1% on a year-to-date basis. (See Pfizer stock analysis on TipRanks)
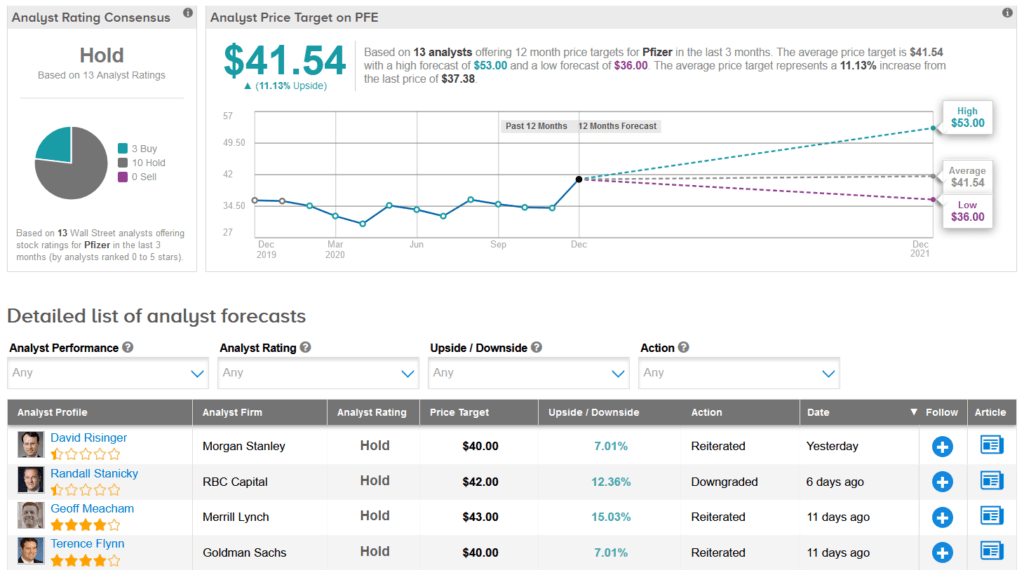
Last week, RBC Capital analyst Randall Stanicky downgraded PFE to Hold from Buy and lowered the price target to $42 from $43, as he argues that the share price already reflects recent COVID-19 vaccine approval milestones, which makes additional upside harder to justify.
“We see it as likely rangebound near-term,” Stanicky wrote in a note to investors, adding that fierce competition from other drugmakers in the COVID-19 vaccine space “could catch up” in 2021.
Overall, the rest of the Street is sidelined on the stock with a Hold analyst consensus. That’s based on 10 Holds vs. 3 Buys. Looking ahead, the average analyst price target stands at $41.54, putting the upside potential at about 11% over the next 12 months.
Related News:
Moderna’s Covid-19 Vaccine Wins FDA Emergency Use Approval
Mesoblast Fails to Meet Primary Endpoint in COVID-19 Trial; Street Sees 29% Upside
FedEx Shares Fall 4% Despite 2Q Earnings Beat; Analysts Stay Bullish