The Pfizer (PFE)-BioNTech (BNTX) COVID-19 vaccine has been authorized for emergency use by the U.S. Food and Drug Administration (FDA) for children aged five through 11 years old.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
The dose provided by healthcare companies can be administered in a two-dose regimen of 10-µg doses in the interval of 21 days. Notably, this is the first COVID-19 vaccine to be approved in the U.S. for children of this age.
The U.S. regulator’s decision was based on the data from Phase 2/3 trials that included around 4,500 children. Results demonstrated that the vaccine reflects a favorable safety profile, robust immune responses and a vaccine efficacy rate of 90.7% in individuals without prior SARS-CoV-2 infection.
Pfizer and BioNTech have also submitted requests for COVID-19 vaccine authorization in this age group to other regulators globally, including the European Medicines Agency. (See Analysts’ Top Stocks on TipRanks)
Initial data from the ongoing Pfizer-BioNTech clinical trial of the vaccine in children of two to less than five years, and those of six months to less than two years of age, are likely to be revealed in the fourth quarter of 2021 or the first quarter of 2022.
Pfizer CEO Albert Bourla said, “Over 6 million children in the U.S. have been diagnosed with COVID-19 since the start of this pandemic, and a high number of young people continue to be infected every week. With this FDA authorization, we have achieved another key marker in our ongoing effort to help protect families and communities, and to get this disease under control.”
Wall Street’s Take
Recently, Mizuho Securities analyst Vamil Divan maintained a Hold rating on Pfizer, and a price target of $43 (1.7% downside potential).
Divan’s neutral stance followed his concerns on the company’s ability to navigate upcoming patent losses, but the analyst awaits continued progress from Pfizer’s pipeline and business development activities.
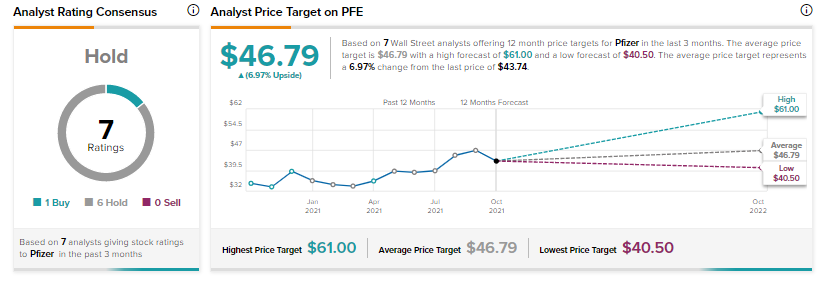
Overall, Pfizer has a Hold consensus rating based on six Holds versus one Buy. The average Pfizer price target of $46.79 implies 7% upside potential to current levels. Shares have increased 32.6% over the past 12 months.
On October 22, Deutsche Bank analyst Emmanuel Papadakis initiated coverage of BioNTech with a Hold rating, and a price target of $250 (10.3% downside potential).
Papadakis believes that though the company has emerged as one of two pioneering mRNA technology companies from the pandemic, its valuation is “unequivocally rich.”
The rest of the Street is cautiously optimistic about BioNTech and has a Moderate Buy consensus rating based on three Buys and seven Holds. The average BioNTech price target of $336.10 implies 20.6% upside potential. Shares have gained 220% over the past 12 months.

Related News:
Boeing Reports Wider-than-Expected Q3 Loss
Alphabet Outperforms in Q3, Google Cloud & YouTube Ad Revenue Falter
Ocugen Submits IND Application for COVAXIN
5 Top Stocks for Q4 2021









