Pharmaceutical giant Pfizer Inc. (PFE) announced that it has applied for emergency use authorization (EUA) with the U.S. Food and Drug Administration (FDA), for its novel COVID-19 oral pill.
The company also signed a licensing agreement with the Medicines Patent Pool (MPP) to allow the use of its COVID-19 pill in low and-middle-income countries.
Pfizer currently has a market cap of $278.68 billion and its shares have gained 37.6% over the past year.
See Analysts’ Top Stocks on TipRanks >>
EUA Approval
Pfizer has applied to the U.S. FDA for the EUA of its investigational oral antiviral pill, PAXLOVID™ (PF-07321332; ritonavir), for the treatment of mild to moderate COVID-19 infection in adults aged 18 years and above, who face an increased risk of hospitalizations or death.
The submission includes clinical data from Pfizer’s Phase 2/3 Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients (EPIC-HR) interim analysis.
The study proves that the pill provides an 89% reduction in risk of COVID-19-related hospitalization or death in patients, from any cause, compared to a placebo within three days of symptom onset, with no deaths in the treatment group.
If approved, Paxlovid can be used as an at-home treatment and as a first measure of controlling the virus’s intensity, and avoiding severe illness. It would be the first oral antiviral pill of its kind, a 3CL protease inhibitor, specifically designed to combat SARS-CoV-2.
Additionally, Pfizer has committed to investing up to $1 billion in the manufacturing and distribution of this investigative treatment pill.
Similarly, Pfizer has also applied for rolling submissions in other countries including the United Kingdom, Australia, New Zealand, and South Korea, and plans to submit to other agencies worldwide.
Management Comments
Albert Bourla, Chairman and CEO of Pfizer said, “The overwhelming efficacy achieved in our recent clinical study of PAXLOVID, and its potential to help save lives and keep people out of the hospital if authorized, underscores the critical role that oral antiviral therapies could play in the battle against COVID-19.”
Bourla added, “We are moving as quickly as possible in our effort to get this potential treatment into the hands of patients, and we look forward to working with the U.S. FDA on its review of our application, along with other regulatory agencies around the world.”
MPP License Agreement
Pfizer also announced that it has signed a licensing agreement with MPP as part of its strategy towards equitable access to COVID-19 vaccines and treatments for all people.
MPP is a United Nations-backed public health organization that aims to increase access to life-saving drugs, particularly to low and-middle-income countries. Through the voluntary agreement, MPP will ensure additional production and distribution of the pill to qualified generic medical manufacturers worldwide. Around 95 countries form part of this agreement, which accounts for about 53% of the world’s population.
Moreover, untill the time COVID-19 remains classified as a Public Health Emergency of International Concern by the World Health Organization (WHO), Pfizer will not receive any royalties from low-income countries and will also waive royalties on sales in all countries.
Commenting on the agreement, Charles Gore, Executive Director of MPP said, “This license is so important because, if authorized or approved, this oral drug is particularly well-suited for low- and middle-income countries and could play a critical role in saving lives, contributing to global efforts to fight the current pandemic.”
Target Price
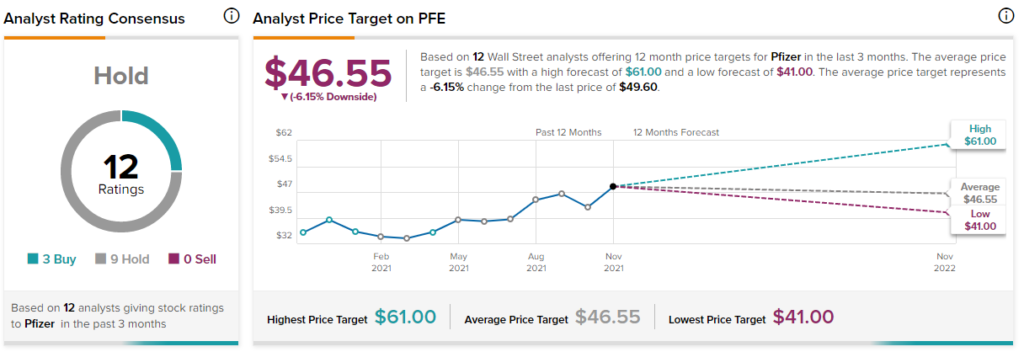
The Wall Street community has a Hold consensus rating on the PFE stock based on 3 Buys and 9 Holds. The average Pfizer price target of $46.55 implies 6.15% downside potential to current levels.
Related News:
Oatly Plunges 21% After Q3 Revenue Miss
Why did Marathon Digital Crash 27% Yesterday?
ExxonMobil launches Asset Sale to Pay Off Debt
















