Pfizer and its German partner BioNTech are planning to offer the first dose of its investigational COVID-19 vaccine by March 1, 2021 to those volunteers who received a placebo in its vaccine trial, Reuters reported.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
Notably, Pfizer (PFE) and BioNTech’s investigational COVID-19 vaccine has not been approved or licensed by the U.S. Food and Drug Administration (FDA). However, on Dec. 11, the U.S. FDA authorized Pfizer-BioNTech to provide the investigational vaccine for emergency use in individuals aged 16 years and older.
Now, Pfizer and BioNTech (BNTX) want the trial participants “who learn they received the placebo, to have the option to receive the investigational vaccine while staying in the study,” the companies said on their website.
The trial participants, who received the placebo, will have two doses of the investigational vaccine within the study. They will “receive a second dose of the investigational vaccine about 21 days later and follow an updated study schedule that includes follow-up and illness visits,” the companies commented. (Compare coronavirus stocks)
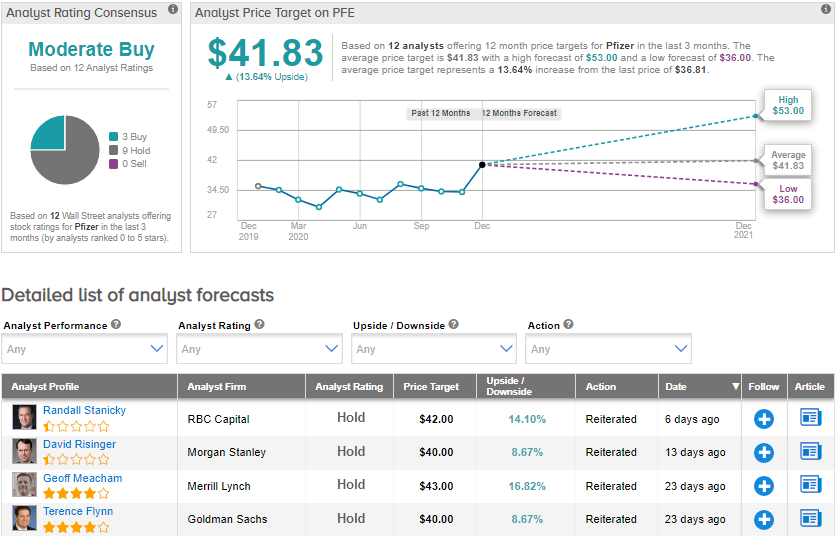
On Dec. 11, Merrill Lynch analyst Geoff Meacham raised the price target for Pfizer stock from $42 to $43 (16.8% upside potential) and maintained a Hold rating. The analyst remains concerned about the company’s patent expirations.
From the rest of the Street, the stock scores a cautiously optimistic analyst consensus of a Moderate Buy based on 3 Buys and 9 Holds. The average analyst price target of $41.83 implies upside potential of about 13.6% to current levels. The stock has declined 6.1% in 2020.
Related News:
Pfizer, BioNTech Ink $1.95B Covid-19 Vaccine Supply Deal With US
RedHill Releases ‘Positive’ Results From Early Covid-19 Trial With Opaganib; Shares Drop 5.6%
U.K. Health Service Extends Window For Second Dose of Pfizer COVID-19 Vaccine









