Novavax, Inc. (NASDAQ: NVAX), one of the leading Biotech stocks, and Serum Institute of India Pvt. Ltd. (SII), revealed that Novavax’s recombinant nanoparticle protein-based COVID-19 vaccine with Matrix-M adjuvant has been authorized by the Drugs Controller General of India (DCGI) for emergency use. The vaccine will be manufactured and marketed in India by SII under the brand name Covovax.
Notably, SII is the world’s largest vaccine manufacturer by volume.
Supporting Data
The regulator’s approval was based on Phase 3 clinical data, which demonstrated that the protein-based COVID-19 vaccine showed more than 90% efficacy and a favorable safety profile.
CEO Comments
Novavax CEO Stanley C. Erck commented, “No one is safe until everyone is safe, and today’s authorization marks a vital step for India, where additional vaccine options and millions of doses are needed in the country’s ongoing efforts to control the pandemic. Novavax and SII will not rest in our partnership to deliver our vaccine to those in India and across the globe, as we work to protect the health of people everywhere.”
Prior Approvals
Novavax and SII have recently received Emergency Use Authorization (EUA) for the vaccine in Indonesia and the Philippines, along with Emergency Use Listing (EUL) with the World Health Organization (WHO), and Conditional Marketing Authorization from the European Commission and EUL. The vaccine will be marketed by Novavax as Nuvaxovid.
Additionally, Novavax has completed rolling submissions for the authorization of the vaccine with regulatory agencies in multiple countries worldwide. Furthermore, submission of the complete package to the U.S. Food and Drug Administration (FDA) is expected by the end of the year.
Analysts Recommendation
Recently, J.P. Morgan analyst Eric Joseph maintained a Hold rating and a price target of $209 (34.11% upside potential) on the stock.
Shares of Novavax have rallied 31% over the past year. The stock scores a Strong Buy consensus rating based on 3 Buys versus 1 Hold. The average Novavax price target of $270 implies 73.25% upside potential.
Risk Analysis
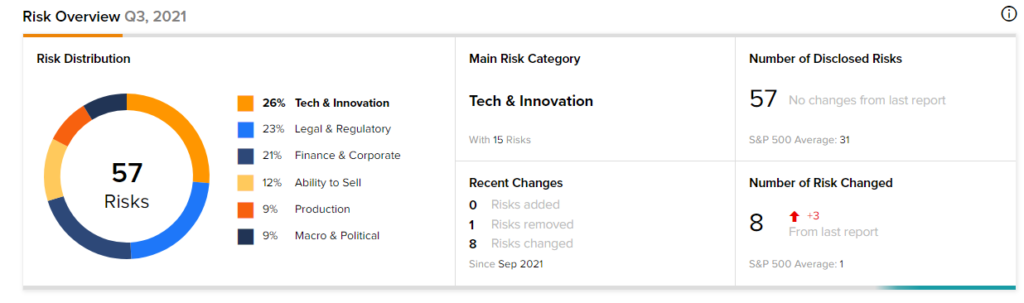
According to the new TipRanks Risk Factors tool, Novavax stock is at risk mainly from three factors: Tech and Innovation, Legal and Regulatory, and Finance and Corporate, which contribute 26%, 23%, and 21%, respectively, to the total 57 risks identified for the stock.
Related News:
BridgeBio Announces ATTRibute-CM Month 12 Primary Endpoint Miss; Shares Plunge 72%
Zoom Acquires Event Solution Assets from Liminal
Swiss Government to Purchase Additional Moderna Booster Vaccines