Novavax, Inc. (NVAX) and Serum Institute of India Pvt. Ltd. (SII) have revealed that Novavax’s recombinant nanoparticle protein-based COVID-19 vaccine with Matrix-M adjuvant has been authorized for emergency use by the National Agency of Drug and Food Control of the Republic of Indonesia.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
Following the news, shares of the biotechnology company rose 15.87% to close at $172.45 on Monday.
The regulator’s decision was based on the data from the Phase 3 clinical trial, which reflected a favorable efficacy and safety profile. The vaccine will be manufactured by SII, the largest vaccine manufacturer by volume globally, in India and marketed by SII in Indonesia under the brand name COVOVAX.
See Analysts’ Top Stocks on TipRanks >>
The CEO of Novavax, Stanley C. Erck, said, “The first authorization of Novavax’s COVID-19 vaccine exemplifies our commitment to equitable global access and will fill a vital need for Indonesia, which despite being the fourth most populous nation on earth, continues to work to procure sufficient vaccine for its population.”
Markedly, the companies have filed for the authorization of Novavax’s COVID-19 vaccine in India and the Philippines, along with Emergency Use Listing (EUL) with the World Health Organization (WHO). (See Novavax stock charts on TipRanks)
Additionally, Novavax has completed rolling submissions for the authorization of the Novavax vaccine with regulatory agencies in the United Kingdom, the European Union, Canada and Australia. Furthermore, submission of the complete package to the U.S. Food and Drug Administration (FDA) is expected by the end of the year.
Wall Street’s Take
Following the company’s updates, B.Riley Financial analyst Mayank Mamtani maintained a Buy rating on the stock with a price target of $305 (76.86% upside potential).
Mamtani said, “The updates are largely consistent with NVAX’s guidance in recent communication on regulatory filings’ progress with initial focus on Asian countries, which remain challenged by access to highly efficacious vaccine products, while also ensuring utmost quality in doses produced, released, and delivered that remains a core requirement of Western economies, notably U.K., EU, Canada and U.S.”
Consensus among analysts is a Strong Buy based on 3 unanimous Buys. The average Novavax price target of $290.33 implies 68.36% upside potential to current levels. Shares have skyrocketed 109.28% over the past year.
Risk Analysis
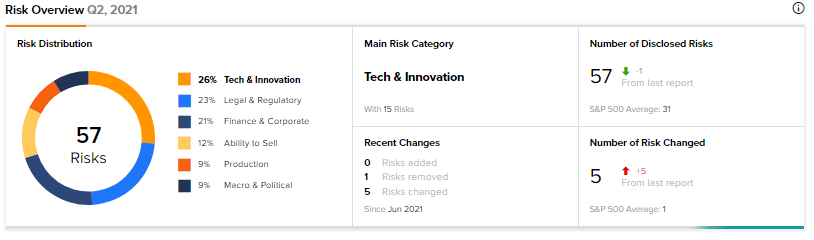
According to the new TipRanks’ Risk Factors tool, the Novavax stock is at risk mainly from three factors: Tech and Innovation, Legal and Regulatory and Finance and Corporate, which contribute 26%, 23% and 21%, respectively, to the total 57 risks identified for the stock.
Related News:
Bristol Myers’ Q3 Earnings Beat but Revenues Miss Estimates
Ocugen Submits IND Application for COVAXIN
Apple Drops 3.5% as Q4 Revenues Disappoint, Supply Crunch Hurts









