Biotechnology company Novavax, Inc. (NASDAQ: NVAX) and Serum Institute of India Pvt. Ltd. (SII) revealed on Friday that NVX-CoV2373, Novavax’s recombinant nanoparticle protein-based COVID-19 vaccine with Matrix-M adjuvant, has been granted Emergency Use Listing (EUL) by the World Health Organization (WHO).
Maximize Your Portfolio with Data Driven Insights:
- Leverage the power of TipRanks' Smart Score a data driven tool to help you uncover top performing stocks and make informed investment decisions.
- Monitor your stock picks and compare them to top Wall Street Analysts' recommendations with Your Smart Portfolio
The vaccine is expected to actively immunize individuals aged 18 years and above against the coronavirus disease 2019 caused by SARS-CoV-2. Notably, an additional EUL filing for Nuvaxovid, the vaccine to be marketed by Novavax, is still undergoing review with the WHO.
Following the news, shares of the company rose 11.5% to close at $217.32 on Friday.
Supporting Data
According to the WHO, Novavax’s COVID-19 vaccine has met the standards for quality, safety, and efficacy required by the organization for getting EUL. The decision followed the review of preclinical, manufacturing, and clinical trial data.
Markedly, positive safety and efficacy data from Novavax’s two pivotal Phase 3 trials supported the decision. Notably, the PREVENT-19 trial included 30,000 participants in the U.S. and Mexico, while another trial included over 14,000 participants in the U.K, demonstrating a well-tolerated profile.
Official Comments
Novavax CEO Stanley C. Erck commented, “Today’s decision from the World Health Organization is vital to ensuring global access to a protein-based COVID-19 vaccine for hundreds of millions of people around the world…We believe this vaccine will help overcome barriers to vaccine access in many regions of the world by leveraging the traditional refrigeration used in existing vaccine supply channels, while also offering an option based on a familiar and well-understood technology.”
“COVOVAX is the first protein-based COVID-19 vaccine option, with demonstrated efficacy and a well-tolerated safety profile, to be made available through the COVAX Facility,” said Adar Poonawalla, the CEO of SII, the largest vaccine manufacturer by volume, globally.
Prior Approvals
Novavax and SII have recently received Emergency Use Authorization (EUA) for COVOVAX in Indonesia and the Philippines and have completed rolling submissions for the authorization of the Novavax vaccine with several regulatory agencies, globally. Furthermore, submission of the complete package to the U.S. Food and Drug Administration (FDA) is expected by the end of the year.
Wall Street’s Take
Following the update, B.Riley Financial analyst Mayank Mamtani reiterated a Buy rating and a price target of $305 (40.35% upside potential) on the stock.
Mamtani commented, “WHO EUL approval is a bigger deal than you think.”
“In light of the Omicron wave sweeping around the globe, we anticipate a slew of regulatory approvals in coming weeks. EMA approval could come in the next week,” the analyst added.
The rest of the Street is cautiously optimistic about the stock, with a Moderate Buy consensus rating based on 2 Buys and 1 Hold. The average Novavax price target of $249.67 implies 14.89% upside potential. Shares have gained 75.17% over the past year, versus the S&P500‘s one-year gain of 25%.
Risk Analysis
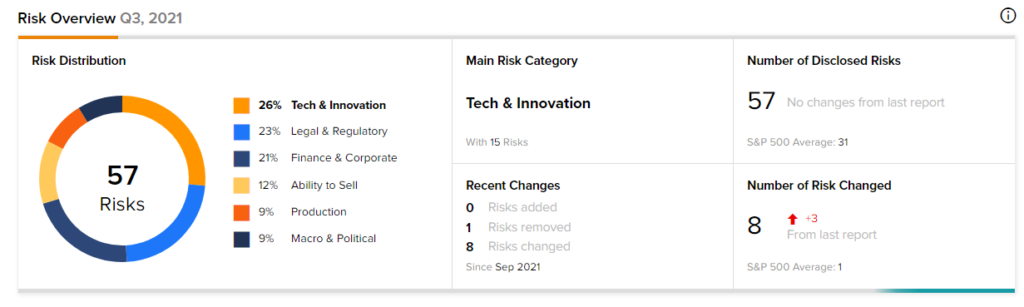
According to the new TipRanks Risk Factors tool, Novavax stock is at risk mainly from three factors: Tech and Innovation, Legal and Regulatory, and Finance and Corporate, which contribute 26%, 23%, and 21%, respectively to the total 57 risks identified for the stock.
Related News:
Adobe Drops 10% on Disappointing Guidance Despite Q4 Revenue Beat
FedEx Posts Better-than-Expected Q2 Results; Shares Gain
Pfizer & BioNTech Files BLA for Approval of COVID-19 Vaccine in Age Group 12-15









