Biotech firm Novavax, Inc. (NASDAQ: NVAX) and Serum Institute of India Pvt. Ltd. (SII) have filed for emergency use authorization (EUA) of Novavax’s recombinant nanoparticle protein-based COVID-19 vaccine with Matrix-M adjuvant to the South African Health Products Regulatory Agency (SAHPRA).
Upon obtaining approval, the vaccine will be manufactured and marketed in South Africa by SII under the brand name Covovax. Notably, SII is the world’s largest vaccine manufacturer by volume.
Following the disclosure of the regulatory submission, shares of Novavax climbed more than 6.5% to close at $134.99 on Monday.
Supporting Data
The supporting documents for EUA of NVX-CoV2373 in South Africa include data from the two pivotal Phase 3 clinical trials. The first trial, PREVENT-19, enrolled around 30,000 participants in the U.S. and Mexico, while in the second trial, almost 15,000 individuals participated in the U.K. In both trials, the vaccine showed superior efficacy with a favorable safety profile.
CEO Comments
Novavax CEO Stanley C. Erck commented, “Novavax is thankful for our long-standing history of partnership in South Africa to advance much-needed vaccines. This is exemplified by the country’s vital role in the Phase 2b clinical trial and booster study of our protein-based COVID-19 vaccine.”
“Novavax and Serum Institute remain focused on delivering the COVID-19 vaccine – built on well-understood technology – where it is needed most. We look forward to SAHPRA’s review and, if authorized, delivering the vaccine to help South Africa control the pandemic,” Erck added.
Prior Approvals
Altogether, Novavax’s vaccine has received authorizations in over 170 countries, including conditional marketing authorization in the European Union and emergency use listing (EUL) from the World Health Organization (WHO). Novavax and SII have recently received EUA for the vaccine in India, Indonesia, and the Philippines.
Additionally, Novavax has completed rolling submissions for the authorization of the Novavax vaccine with regulatory agencies in multiple countries worldwide. Furthermore, the complete package was submitted to the U.S. Food and Drug Administration (FDA) at the end of 2021.
Analyst Recommendations
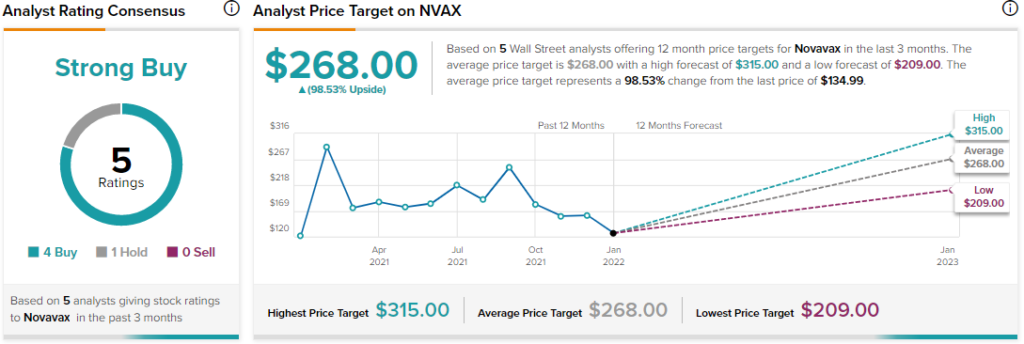
Recently, B.Riley Financial analyst Mayank Mamtani maintained a Buy rating on Novavax and lifted the price target to $315 (133.35% upside potential) from $305.
Shares of Novavax have rallied 8.7% over the past year. The stock scores a Strong Buy consensus rating based on 4 Buys versus 1 Hold. The average Novavax price target of $268 implies 98.53% upside potential.
Download the TipRanks mobile app now
To find good ideas for stocks trading at attractive valuations, visit TipRanks’ Best Stocks to Buy, a newly launched tool that unites all of TipRanks’ equity insights.
Read full Disclaimer & Disclosure
Related News:
Take-Two to Acquire Zynga for $12.7B
Ocugen Reveals Positive Results from Phase 2 Analysis of COVAXIN
FedEx Operations Affected by Omicron & Bad Weather – Report
Questions or Comments about the article? Write to editor@tipranks.com