Biotechnology company Novavax, Inc. (NASDAQ: NVAX) revealed that the first booster doses of NVX-CoV2373, Novavax’s recombinant nanoparticle protein-based COVID-19 vaccine with Matrix-M adjuvant, has been administered under the company’s ongoing PREVENT-19 pivotal Phase 3 clinical trial. The study is designed to evaluate the safety and efficacy of the heterologous or homologous third dose of the vaccine.
Maximize Your Portfolio with Data Driven Insights:
- Leverage the power of TipRanks' Smart Score, a data-driven tool to help you uncover top performing stocks and make informed investment decisions.
- Monitor your stock picks and compare them to top Wall Street Analysts' recommendations with Your Smart Portfolio
Meanwhile, shares of the company declined 5.4% on Tuesday to close at $191.07.
Supporting Factors
All individuals who participated in the PREVENT-19 trial are now eligible for a third booster dose of NVX-CoV2373. The booster dose is similar to the vaccine administered in the two-dose primary vaccinations (5 micrograms of recombinant Spike protein plus 50 micrograms of Matrix-M adjuvant). Notably, the booster dose can be taken after a minimum of six months post receipt of the active vaccine.
Novavax’s action followed the recommendations of the World Health Organization (WHO) Strategic Advisory Group of Experts on Immunization (SAGE) for the primary two-dose vaccination series of NVX-CoV2373 in persons aged 18 years and older. Also, the additional third dose of the vaccine is recommended to immunocompromised persons.
Notably, the recommendations followed the Emergency Use Listing (EUL) of the vaccine by WHO.
Official Comments
Novavax CEO Stanley C. Erck commented, “This interim recommendation from WHO provides helpful guidance for the use of our COVID-19 vaccine as countries that rely on EUL begin their own assessment and underscores the critical role that we expect the vaccine will play in the global fight against the coronavirus. We look forward to delivering our recombinant protein-based vaccine to hundreds of millions of people around the world in partnership with our partner, Serum Institute of India.”
Wall Street’s Take
Recently, H.C. Wainwright analyst Vernon Bernardino reiterated a Buy rating and a price target of $294 (53.87% upside potential) on the stock.
Shares of Novavax have rallied 65.6% over the past year. The stock scores a Strong Buy consensus rating based on 3 Buys versus 1 Hold. The average Novavax price target of $270 implies 41.31% upside potential.
Risk Analysis
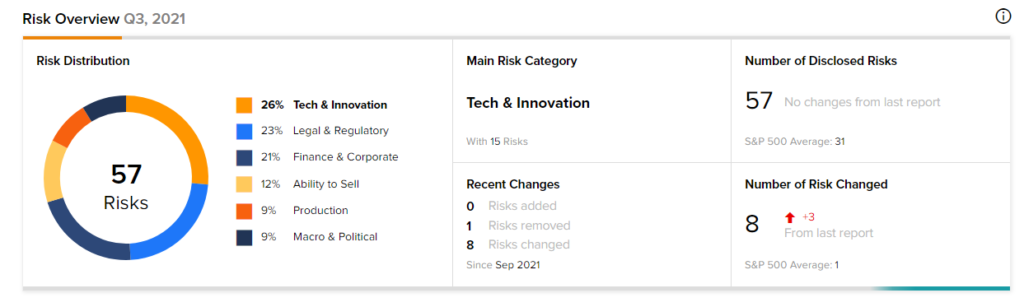
According to the new TipRanks Risk Factors tool, Novavax stock is at risk mainly from three factors: Tech and Innovation, Legal and Regulatory, and Finance and Corporate, which contribute 26%, 23%, and 21%, respectively to the total 57 risks identified for the stock.
Related News:
Novavax Receives Second EUL from WHO for Nuvaxovid
Pfizer & BioNTech SE to Supply Over 200 Additional Doses of COMIRNATY
McKesson to Vend Austrian Business