Health Canada has authorized the booster dose of Moderna, Inc.’s (MRNA) COVID-19 vaccine, Spikevax.
The dose provided by the American pharmaceutical and biotechnology company can be administered at the 50 µg level to people aged 18 and above. Notably, the dose should be administered minimum after six months following the primary vaccinations.
Health Canada’s authorization was based on the scientific evidence provided by the company, which included a data analysis from the Phase 2 clinical study of mRNA-1273. Notably, the analysis was amended to offer a booster dose of mRNA-1273 at the 50 µg dose level to interested participants 6-8 months following their second dose, the company said.
CEO Comments
The CEO of Moderna, Stephane Bancel, said, “We would like to thank Health Canada for this authorization and the Government of Canada for its continued confidence in our mRNA platform. We are grateful for the opportunity to provide Canadians with another layer of protection.”
See Top Smart Score Stocks on TipRanks >>
Prior Approvals
Recently, Swissmedic authorized the booster dose of Spikevax to people aged 12 and above at the same dose level. The third dose was also approved at the 100 µg dose level for people with a weakened immune system, a minimum of 28 days after the second dose.
Additionally, the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has also recommended the booster dose of Spikevax in the European Union for people aged 18 years and above.
It followed the recent U.S. Food and Drug Administration’s (FDA) emergency use authorization (EUA) for the booster dose in the U.S. (See Moderna stock charts on TipRanks)
Wall Street’s Take
Recently, Piper Sandler analyst Edward Tenthoff reiterated a Buy rating and a price target of $348 (48% upside potential) on the stock.
Tenthoff believes that Moderna’s mRNA pipeline is going well but the COVID-19 vaccine “remains the primary near-term driver.”
Overall, the stock has a Hold consensus rating based on 6 Buys, 4 Holds and 3 Sells. The average Moderna price target of $310.27 implies 32% upside potential from current levels. Shares have gained 46.5% over the past six months.
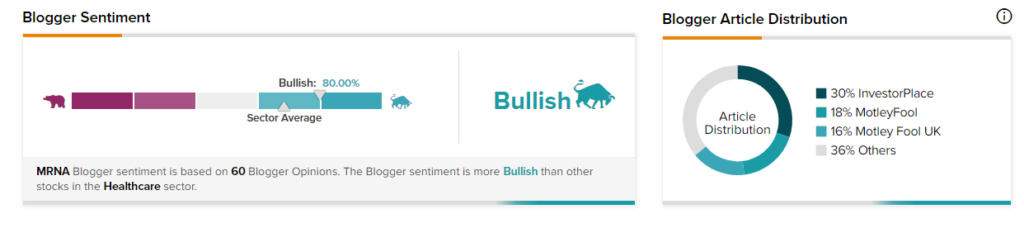
Bloggers Weigh In
TipRanks data shows that financial blogger opinions are 80% Bullish on MRNA, compared to a sector average of 68%.
Related News:
Gain Therapeutics Reports Quarterly Loss; Shares Rise
Moderna Presents Data from Phase 1 Clinical Study of mRNA Triplet Program
Boeing to Initiate Three New Freighter Conversion Lines



















