Moderna announced that Canada has authorized the use of its COVID-19 vaccine for the immunization of people aged 18 years and older. Shares closed 3.5% higher on Wednesday.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
Specifically, Moderna (MRNA) said that Health Canada has approved its mRNA-1273 vaccine under an interim order for the import and sale of COVID-19 drugs and is based on a rolling review of data, which was released on October 12. With this approval, Canada is now the second country to authorize Moderna’s vaccine following the emergency-use authorization by the US Food and Drug Administration earlier this month.
“I want to thank Health Canada and the Canadian government for this authorization, which is a significant moment in our company’s history,” said Moderna CEO Stéphane Bancel. “Health Canada provided a comprehensive, thorough review and provided us with ongoing guidance as we worked together to achieve this authorization.”
Earlier this month, the Canadian government exercised its option to double its order of Moderna COVID-19 vaccine doses to 40 million doses.
Moderna’s mRNA-1273, which is undergoing late-stage trials, triggers an antibody response specifically to the SARS-CoV-2 spike protein, and has demonstrated efficacy of 94.1% against COVID-19.
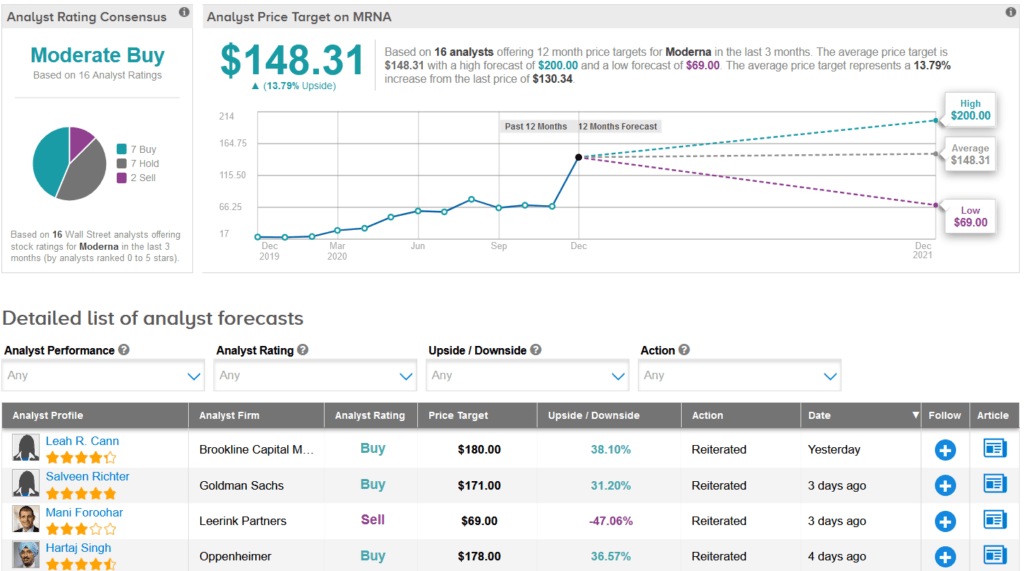
Shares of Moderna have surged 29% over the past month and are up a staggering 566% so far this year. Meanwhile, Wall Street analysts still have a cautiously optimistic outlook on the stock with a Moderate Buy consensus. What’s more, the average price target stands at $148.31, indicating another 14% upside potential lies ahead.
In a more bearish outlook, Leerink analyst Mani Foroohar reiterated a Sell rating on MRNA shares along with a $69 price target (47% downside potential), following the vaccine approval by the FDA.
The analyst expects the stock to “remain volatile as investor debates on commercial and execution risk come to the fore.”
“As we are in the midst of a global buildout of capacity analogous to a Manhattan project for vaccines,” Foroohar wrote in a note to investors, “We see excess capacity, high competitive intensity, and limited pricing power as likely long-term structural features of vaccine end-markets, presenting secular challenges to a sub-scale player such as MRNA.” (See MRNA stock analysis on TipRanks).
Related News:
Sorrento Pops 8% Pre-Market On FDA Application For Covid-19 Test
Polaris Appoints Interim CEO, CFO Effective Jan. 1; Street Sees 14% Upside
HeidelbergCement Looks to Divest California Operations – Report









