Moderna announced that it is in talks to potentially supply the government of South Korea with 40 million or more doses of its COVID-19 vaccine. Shares gained 2.4% in Wednesday’s pre-market trading.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
Under the terms of the proposed agreement, distribution of Moderna’s (MRNA) mRNA-1273 vaccine would begin in the second quarter of 2021. On Tuesday, the Yonhap news agency reported that the US biotech company had agreed to supply coronavirus vaccine doses for 20 million people in South Korea starting in the second quarter of next year, according to the South Korean presidential office.
The confirmation of the talks comes just a day after Moderna secured a $1.97 billion contract from the US army to manufacture an additional 100 million doses of mRNA-1273 by June 30, 2021.
Moderna’s mRNA-1273, which is undergoing late-stage trials, triggers an antibody response specifically to the SARS-CoV-2 spike protein, and has demonstrated efficacy of 94.1% against COVID-19. The vaccine has been granted emergency-use authorization by the US Food and Drug Administration, and Canada has authorized its use for the immunization of people aged 18 years and older.
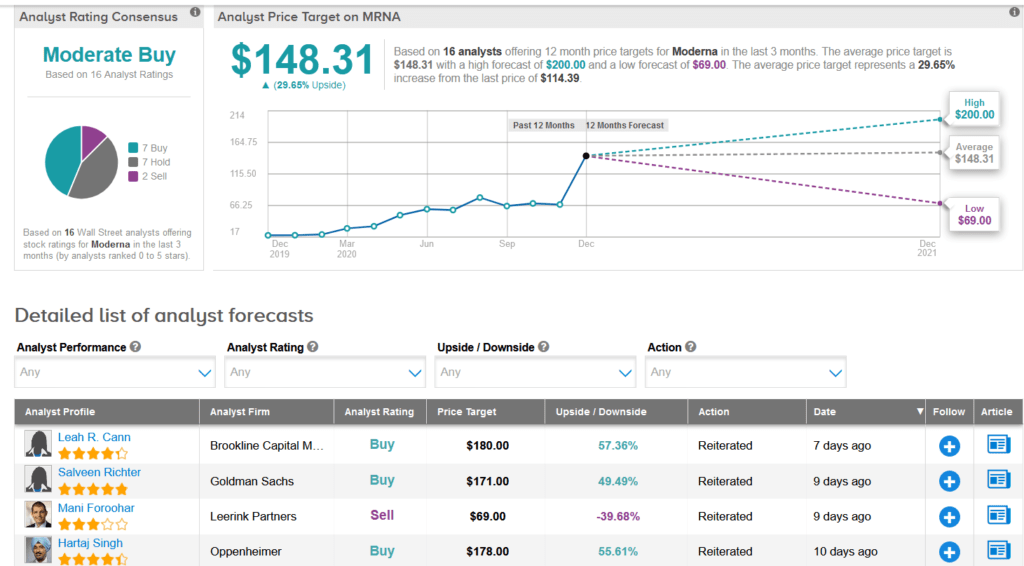
Shares of Moderna have dropped 25% over the past month but are still up a staggering 485% on a year-to-date basis. Meanwhile, Wall Street analysts still have a cautiously optimistic outlook on the stock with a Moderate Buy consensus. What’s more, the average price target stands at $148.31, indicating another 30% upside potential lies ahead.
In a more bearish outlook, Leerink analyst Mani Foroohar reiterated a Sell rating on MRNA shares along with a $69 price target (40% downside potential), following the FDA’s vaccine approval.
The analyst expects the stock to “remain volatile as investor debates on commercial and execution risk come to the fore.”
“We are in the midst of a global buildout of capacity analogous to a Manhattan project for vaccines,” Foroohar wrote in a note to investors. “We see excess capacity, high competitive intensity, and limited pricing power as likely long-term structural features of vaccine end-markets, presenting secular challenges to a sub-scale player such as MRNA.” (See MRNA stock analysis on TipRanks).
Related News:
Hepion Pops 47% Amid ‘Positive’ Trial Data From Fatty Liver Disease Treatment
Pfizer, BioNTech to Deliver Additional 100M Vaccine Doses to EU
Quanterix Wins FDA Approval For Covid-19 Antibody Test; Shares Rise 6%









