Medtronic (MDT) has announced positive results from its US pivotal trial of its MiniMed 780G Advanced Hybrid Closed Loop (AHCL) insulin pump at the virtual American Diabetes Association (ADA) conference.
The investigational MiniMed 780G system is designed to automate the delivery of both base-level insulin and correction doses to help people with diabetes avoid highs and lows.
It features a default target of 100mg/dL (with the option of 120mg/dL), programmable insulin action time from two to eight hours, and automatic corrections every five minutes.
Results of the 90-day at home US pivotal trial, studying adults and adolescents aged 14-75 years old, show the trial successfully met both safety and glycemic endpoints and demonstrated no occurrences of severe adverse events.
Overall Time in Range (defined as 70-180 mg/dL) reached 75%, with overall Time Below Range (defined as less than 70 mg/dL) at just 1.8%. That was alongside an average A1C blood sugar level of 7.0%, with autocorrection contributing to 22% of all bolus insulin and participants being in SmartGuard (closed loop) 95% of the time. Mean sensor glucose (SG) was at 148 mg/dL overall, and 144 mg/dL at the default 100mg/dl target.
Results from a study questionnaire also demonstrated high user satisfaction with 96% indicating it was easy to use.
Lastly, the lower target glucose and active insulin time (AIT) settings substantially improved Time in Range, without increasing hypoglycemia. Time in Range increased to 76%, with a 100 mg/dL target and AIT of two to three hours and reached 79% when AIT was set to two hours.
“Most notable is the additional target glucose level of 100 mg/dL — which will be lower than the other commercially available devices in this category,” cheered Dr. Anders Carlson, investigator of the study. “We often hear from our patients about wanting lower glucose target settings, and there is certainly a desire to see that represented more in the current marketplace.”
Data from a second, randomized cross-over clinical trial based in New Zealand studied more challenging patients, including those with less-controlled diabetes and children as young as seven years old.
The results also demonstrated improved outcomes for those on the Medtronic AHCL system when compared to a predictive low-glucose management (PLGM) algorithm.
The study met primary endpoints (with a 13-point overall improvement in Time in Range to 70.4%), as well as secondary endpoints (decreasing overall time above and below range). Patients also reported being highly satisfied with their experience overall, with 85% agreeing that the system improved their quality of life.
“We wanted to design a system that further simplifies diabetes management and provides an extra layer of protection for the times one may forget a pre-meal bolus or miscalculate their carbohydrates” explained Robert Vigersky, CMO for Medtronic’s Diabetes Group.
Data from the pivotal study will be submitted as part of a Premarket Approval (PMA) application to the U. S. FDA for commercial approval of the MiniMed 780G system, says Medtronic. The MiniMed 780G system received CE (Conformité Européenne) Marking in June 2020.
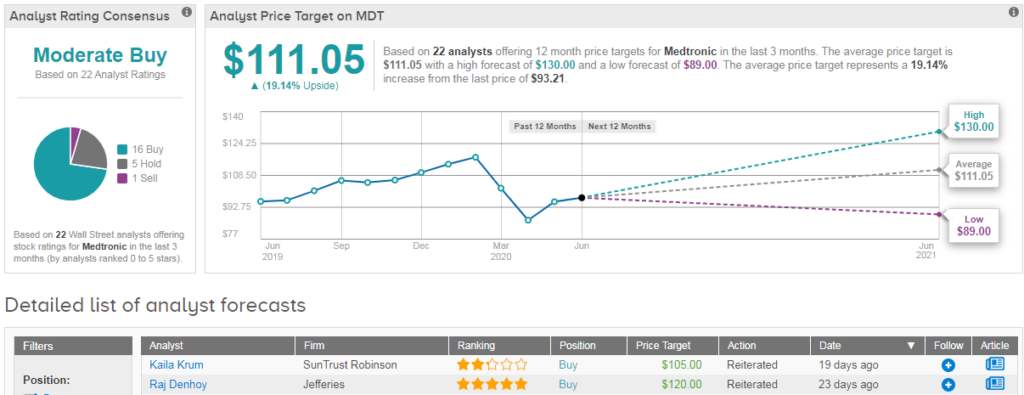
Shares in Medtronic have dropped 18% year-to-date, and analysts have a cautiously optimistic Moderate Buy outlook on the stock. This breaks down into 16 recent buy ratings vs 5 hold ratings and 1 sell. Meanwhile the $111 average analyst price target indicates 19% upside potential from current levels. (See MDT stock analysis on TipRanks).
Related News:
5 Promising Covid-19 Vaccines Picked For Trump’s Operation Warp Speed
AstraZeneca Inks Europe Deal For 400M Covid-19 Vaccine Doses
Sorrento Edges Higher On Debt Repayment As Top Analyst Says Buy
















