Shares of Kala Pharmaceuticals (NASDAQ:KALA) skyrocketed over 60% in after-hours trading today. This can be attributed to its persistent corneal epithelial defect treatment, which got the nod from the FDA to proceed with its development.
The firm will now move on to its phase 2b clinical trial and is likely to recruit 90 patients to study the effectiveness of the new treatment.
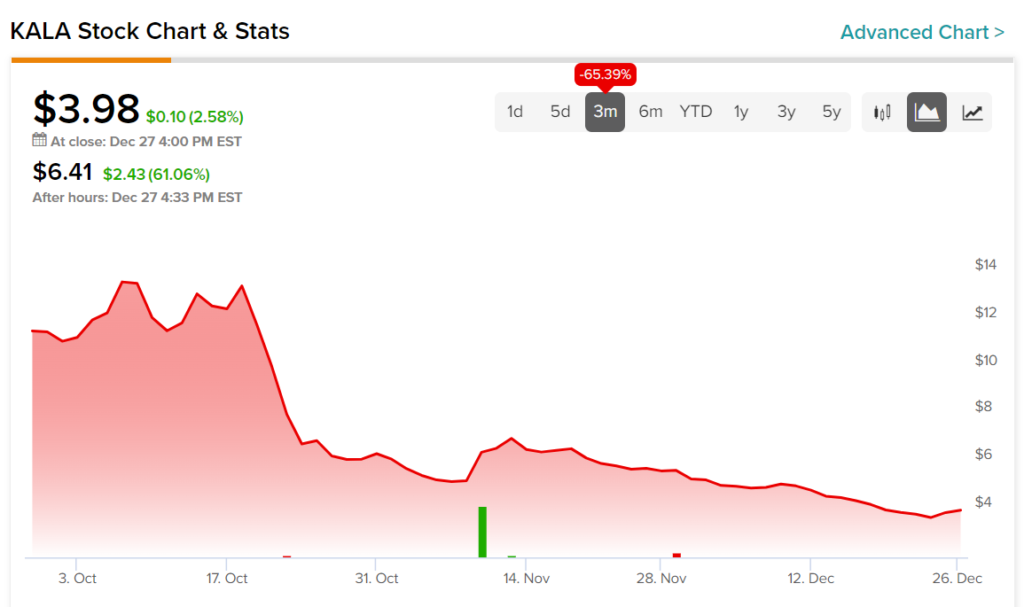
Although more recent investors may be happy with the move, any longer-term investors still have a long way to go before they see any meaningful recovery. Indeed, the stock would still have to double from here to get to its three-month high.
Special end-of-year offer: Access TipRanks Premium tools for an all-time low price! Click to learn more.




















