It’s usually a full approval of a new drug that fires up pharmaceutical companies like Kala Pharmaceuticals (NASDAQ:KALA). But sometimes, even just a minor nod from the FDA can fire up investor enthusiasm. Kala Pharmaceuticals closed up over 218% after Wednesday’s trading session, and after-hours trading kept the streak alive, though not to such an extent.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
More specifically, Kala Pharmaceuticals benefited from the FDA accepting a new kind of application from the company, an IND, or an “investigational new drug” application. Currently, the new drug in question is called KPI-012. It targets an eye disease known as “persistent corneal epithelial defect,” or PCED. PCED is really only a problem for those who suffer a corneal injury; those with PCED may find that their corneas won’t heal properly after such an injury.
With KPI-012, however, addressing and suppressing that defect is possible, and eyes can heal properly. Kala Pharmaceuticals expects to start a phase Iib trial early in 2023. It also managed to net a $25 million investment to drive such testing. A successful test would allow Kala to file for a “biologics license application” after one more successful test. Two such tests are required to take the next step.
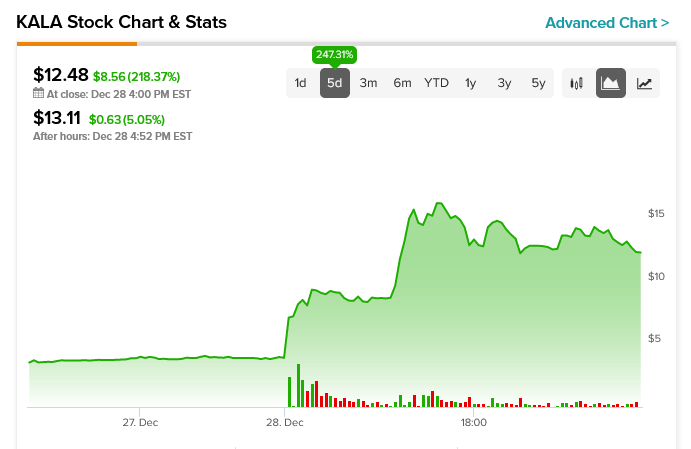
Reports noted that authorities halted trading in KALA six times today, all due to volatility. However, the last five days of trading have been kind to Kala Pharmaceuticals. The stock plateaued at just under $4 a share ahead of the Christmas holiday. However, when trading reopened, the stock exploded upward and was up four-fold from its December 23 price at one point.
Special end-of-year offer: Access TipRanks Premium tools for an all-time low price! Click to learn more.









