The booster dose of Johnson & Johnson’s (JNJ) COVID-19 vaccine has been authorized for emergency use by the U.S. Food and Drug Administration (FDA) for people aged 18 and above. Notably, the dose should be administered minimum after two months following the primary vaccination with a single-shot Johnson & Johnson COVID-19 vaccine.
Invest with Confidence:
- Follow TipRanks' Top Wall Street Analysts to uncover their success rate and average return.
- Join thousands of data-driven investors – Build your Smart Portfolio for personalized insights.
Additionally, the booster dose has also been authorized for eligible individuals, who received a different authorized or approved COVID-19 vaccine. Markedly, the formulation and dosage of the booster shot are similar to the primary shot.
The U.S. regulator’s decision followed a unanimous recommendation from the FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC). The approval was based on the data provided by the company, which included efficacy, safety and immunogenicity data from the clinical trials and real-world evidence data. Notably, following the initial vaccination with the company’s COVID-19 single-shot vaccine, when the primary or booster dose was administered, it safeguarded against symptomatic disease with a high tolerance level.
The CSO of Johnson & Johnson, Paul Stoffels, said, “Our data support a schedule that provides benefit to individuals based on their risks associated with COVID-19, whether administered as a single dose for an efficient response to the pandemic, or as a booster dose after at least two months – to protect against symptomatic COVID-19. We also welcome the FDA’s decision to include a heterologous boosting option as part of this authorization. The ability to boost immune responses regardless of the primary vaccine regimen an individual has received provides more flexibility in protecting those already immunized, and is very beneficial to global public health as we look to curb this pandemic.” (See Johnson & Johnson stock charts on TipRanks)
Recently, Johnson & Johnson reported mixed results for the third quarter of 2021. Quarterly revenues stood at $23.3 billion, up 10.7% year-over-year. The figure, however, missed the Street’s estimate of $23.74 billion. The company reported quarterly earnings of $2.60 per share, which was up 18.2% year-over-year and surpassed the consensus estimate of $2.36 per share.
See Analysts’ Top Stocks on TipRanks >>
For 2021, the company expects revenues to be in the range of $94.1 billion to $94.6 billion. Analysts expect the same to be $94.3 billion. Additionally, earnings are forecast to be in the range of $9.77 to $9.82 per share. The consensus estimate for the same is pegged at $9.66 per share.
Following the third-quarter results, Raymond James analyst Jayson Bedford reiterated a Buy rating on the stock but lowered the price target to $178 (8.68% upside potential) from $183.
Bedford noted “cross-currents” in the company’s earnings report and views Johnson & Johnson’s business to be strong than concerned.
Though the talc headlines might continue to impact investors’ sentiment, it seems manageable based on the company’s recent moves, the analyst added.
The consensus rating among analysts is a Strong Buy based on 6 Buys versus 2 Holds. The average Johnson & Johnson price target stands at $192.17 and implies upside potential of 17.3% to current levels. Shares have gained 13.8% over the past year.
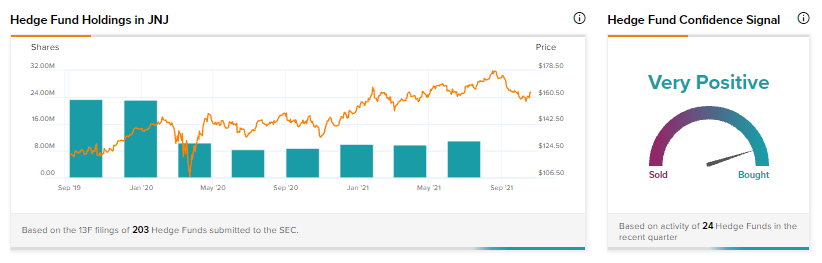
TipRanks’ Hedge Fund Trading Activity tool shows that confidence in Johnson & Johnson is currently Very Positive, as the cumulative change in holdings across all 24 hedge funds that were active in the last quarter was an increase of 1.1 million shares.
Related News:
Synovus’ Q3 Earnings Top Estimates; Shares Gain
Castle Biosciences Inks $30M Deal to Acquire Cernostics; Shares Rise
Atea Reveals Topline Results of Phase 2 MOONSONG Trial; Shares Crash 66%









