Jazz Pharmaceuticals plc (NASDAQ: JAZZ), a global biopharmaceutical company, has revealed that Xywav (calcium, magnesium, potassium, and sodium oxybates) oral solution has been granted Orphan Drug Exclusivity (ODE) by the U.S. Food and Drug Administration (FDA). Xywav is a first-of-its-kind oral solution designed to treat idiopathic hypersomnia, a neurologic sleep disorder in adults.
Maximize Your Portfolio with Data Driven Insights:
- Leverage the power of TipRanks' Smart Score, a data-driven tool to help you uncover top performing stocks and make informed investment decisions.
- Monitor your stock picks and compare them to top Wall Street Analysts' recommendations with Your Smart Portfolio
Supporting Data
Xywav has been approved with multiple dosing options based on the needs of patients. Notably, most individuals who participated in the clinical trials found the twice a night regimen optimal.
Markedly, the medication was granted its first ODE for seven-year market exclusivity in June 2021 for the treatment of cataplexy, or excessive daytime sleepiness (EDS), in patients aged 7 years and older with narcolepsy.
Official Comments
Jazz CMO Robert Iannone commented, “Prior to the approval of Xywav no treatments were approved for people living with this debilitating and unique sleep disorder, so we are very proud of how we advanced the medicine from concept to commercial availability and are encouraged that FDA has recognized Xywav’s impact by granting ODE for the treatment of idiopathic hypersomnia.”
Wall Street’s Take
Recently, Bernstein analyst Aaron Gal maintained a Buy rating on the stock but decreased the price target to $175 (33.58% upside potential) from $200.
Consensus among analysts is a Strong Buy based on 12 Buys versus 1 Hold. The average Jazz price target of $196.50 implies 50% upside potential from current levels. However, shares have lost 19.3% over the past year.
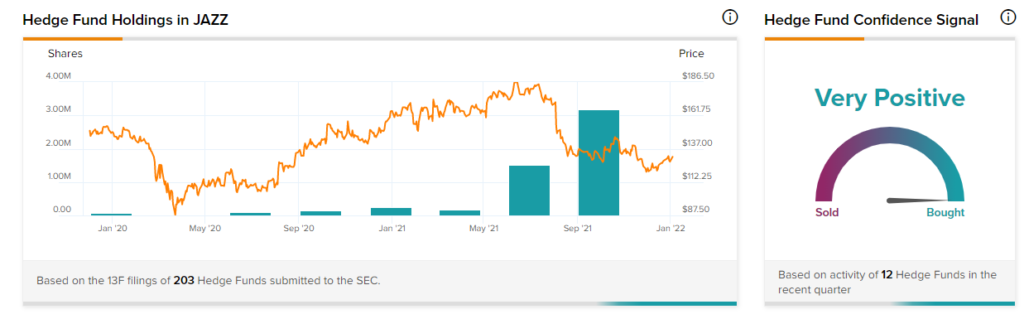
Hedge Funds
TipRanks’ Hedge Fund Trading Activity tool shows that confidence in Jazz is currently Very Positive, as the cumulative change in holdings across all 12 hedge funds that were active in the last quarter was an increase of 1.7 million shares.
Download the mobile app now, available on iOS and Android
Related News:
Tesla Records Over 300,000 Deliveries in Q4, Beats Expectations
Li Auto December Deliveries Jumps 130%
NIO Notifies Shareholders of Convertible Notes Option









