Iovance (NASDAQ:IOVA) stock surged about 33% in Friday’s extended trading session after its advanced melanoma treatment, Amtagvi, received accelerated approval from the U.S. Food and Drug Administration (FDA). It is worth highlighting that Amtagvi is the first tumor-infiltrating lymphocyte (TIL) cell therapy to receive FDA approval for solid tumor cancer.
The regulatory approval was driven by the positive outcomes observed in Amtagvi’s Phase 2 clinical trial. The trial demonstrated a 31.5% response rate among the 73 enrolled patients. Additionally, nearly 50% of the patients showed no signs of tumor progression after 12 months of follow-up.
Remarkably, Iovance is conducting a Phase 3 trial to confirm the clinical benefits of the therapy. Iovance expects TIL therapy to start generating substantial revenue in the second quarter of this year.
Is IOVA a Good Stock to Buy?
The company is testing the potential of TIL in other solid tumor categories, such as metastatic non-small cell lung cancer. Positive developments on this front are expected to propel the company’s stock value even higher.
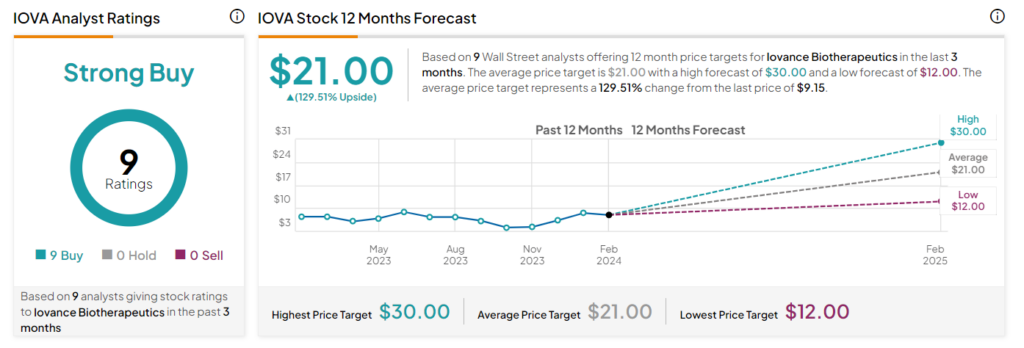
Interestingly, analysts are highly optimistic about the stock. Iovance has a Strong Buy consensus rating based on nine unanimous Buy ratings. IOVA stock has gained more than 67% over the past three months, and the average IOVA price target of $21 implies an upside potential of 129.5% at current levels.