Insmed (NASDAQ:INSM) is making waves in the market with successful results from a Phase 3 trial of potentially a groundbreaking drug in Brensocatib for non-cystic fibrosis bronchiectasis patients. The drug could pioneer a new treatment era for this difficult-to-treat patient population by achieving its primary objective of significant reductions in the annual rate of pulmonary exacerbations, as well as several secondary endpoints,
Invest with Confidence:
- Follow TipRanks' Top Wall Street Analysts to uncover their success rate and average return.
- Join thousands of data-driven investors – Build your Smart Portfolio for personalized insights.
The positive data has propelled Insmed towards a New Drug Application (NDA), aiming for FDA submission in Q4 2024, a subsequent commercial launch by mid-2025, and a further expansion to Europe and Japan in 2026. This promising progress has boosted INSM’s stock by 147% over the past 90 days, with expectations for further upside, making this a compelling biopharma option for investors with higher-risk appetites.

Insmed’s Promising Treatment Candidate
Insmed is a global biopharmaceutical corporation developing and launching treatments for severe and rare illnesses. It currently provides ARIKAYCE, a combination antibacterial drug for treating adults with Mycobacterium avium complex lung disease.
The company recently announced promising results from its Phase 3 ASPEN study of Brensocatib, a drug candidate for non-cystic fibrosis bronchiectasis, a severe lung disease. The study met its primary target, with both tested dosage strengths showing significant reductions in the annual rate of pulmonary exacerbations compared to placebo.
Following these positive results, Insmed intends to submit a New Drug Application (NDA) for Brensocatib to the U.S. Food and Drug Administration (FDA) in Q4 2024. If approved, Brensocatib will be the first treatment for bronchiectasis, which affects about 450,000 patients in the U.S., and the first approved dipeptidyl peptidase 1 (DPP1) inhibitor, a new action mechanism potentially addressing a range of neutrophil-mediated diseases.
Insmed’s Recent Financial Results
The company recently published first-quarter results for 2024. Revenue was $75.5 million, a 16% year-over-year growth from $65.2 million, though short of analysts’ expectations of $78.58 million. The critical driver of revenue growth was ARIKAYCE’s net sales across the U.S., Japan, and Europe. Cost of product revenues increased to $17.5 million, compared to $13.8 million during the corresponding period in 2023. This was primarily due to increased sales volumes of ARIKAYCE. Despite this, Insmed’s net loss of $157.1 million marked a slight improvement over $159.8 million in Q1 2023. While analysts had expected earnings per share at -$1.24, the company exceeded expectations by reporting a higher EPS of -$1.06.
As of 31st March 2024, Insmed reported having cash and cash equivalents of $595.7 million. This should give the firm plenty of runway to bring Brensocatib to market.
Management has given guidance for 2024, with sales projections for its global ARIKAYCE revenues between $340 million and $360 million, signifying a 15% year-over-year growth at the midpoint compared to 2023. Over 80% of Insmed’s total expenditures are expected to be allocated to its mid to late-stage and commercial programs (ARIKAYCE, Brensocatib, and TPIP), with less than 20% of the overall spending on its early-stage research programs.
Is INSM Stock a Buy?
Analysts following the company have been bullish on the stock. For instance, Guggenheim analyst Vamil Divan recently raised the price target on the shares to $95 from $70 while maintaining a Buy rating, noting the “encouraging feedback” from bronchiectasis experts on the strength of the data and how broadly they expect to use Brensocatib in their patients, marking the firm as a “Best Idea” within the coverage universe.
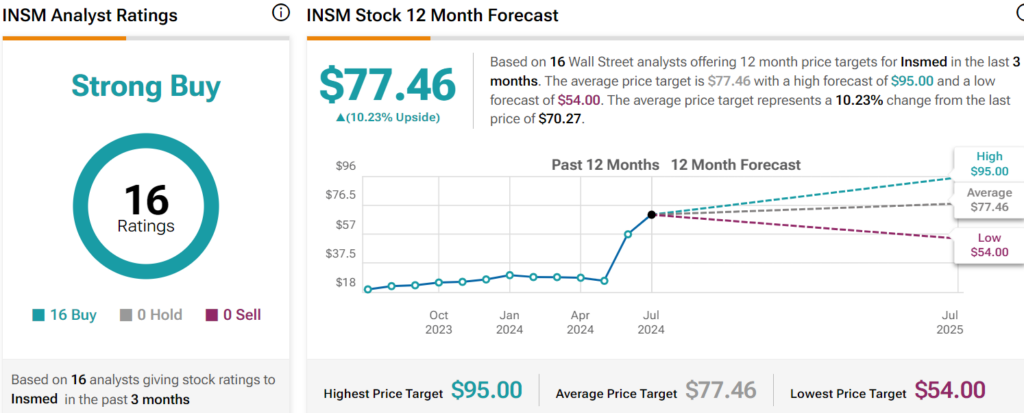
Insmed is rated a Strong Buy overall, based on the recommendations and price targets recently issued by 15 analysts. The average price target for INSM stock is $77.46, representing a potential upside of 10.23% from current levels.
The stock has been on a solid upward trend, climbing over 239% in the past year. Shares trade at the high end of their 52-week price range of $19.74 – $70.65 and continue to show positive price momentum, trading above the 20-day (63.72) and 50-day (51.15) moving averages. The stock looks richly valued, with a P/S ratio of 31.93x compared to the Biotechnology industry average of 10.81, reflecting the growth potential being priced in at this time.
INSM in Summary
Insmed’s successful results in the Phase 3 trial of a leading pipeline candidate have sent the stock soaring. The drug has shown potential in significantly reducing pulmonary exacerbations and appears poised to be the first treatment of its kind on the market. Thus, it presents a unique investment opportunity for those seeking higher-risk, higher-return potential biopharma stocks.









