Shares of Inovio Pharmaceuticals plunged 28.3% on Monday after the biotechnology company announced that the US Food and Drug Administration (FDA) halted the start of the Phase 2/3 trials of its COVID-19 vaccine. The stock dropped another 3.6% in Monday’s extended market session.
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
Inovio (INO) said that the US regulatory body is seeking more information about its vaccine candidate INO-4800, including details of the delivery device used to inject genetic material into cells. The company stated that it would respond to FDA’s queries in October, after which the agency will have up to 30 days to make a decision.
“This partial clinical hold is not due to the occurrence of any adverse events related to INOVIO’s ongoing expanded Phase 1 study of INO-4800, the conduct of which may continue and is not impacted by the FDA’s notification,” the company said in a statement. (See INO stock analysis on TipRanks).
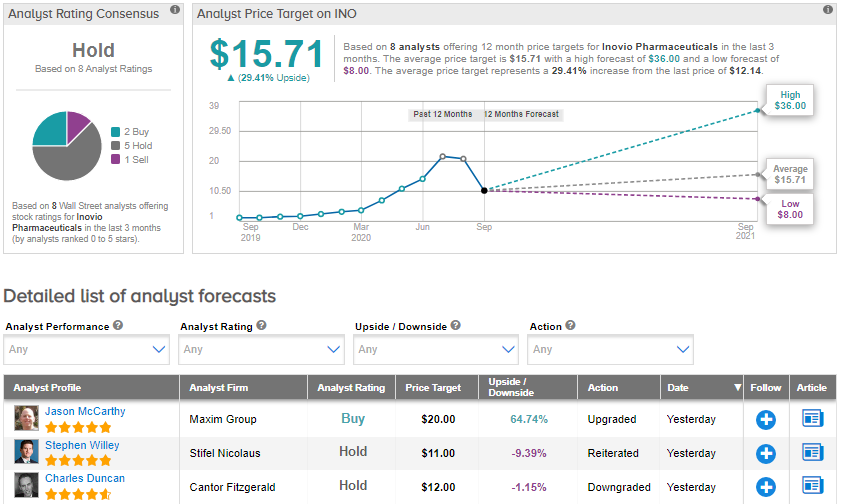
Meanwhile, Maxim Group analyst Jason McCarthy upgraded the stock to Buy from Hold following the FDA action, as he believes that shares have been oversold. McCarthy is confident that the issue with the FDA will be resolved in October or November. The analyst reiterated his price target of $20 (64.7% upside potential).
Overall, the Street is sidelined on the stock. The Hold analyst consensus is based on 5 Holds, 2 Buys and 1 Sell. With shares up nearly 268% year-to-date, the average price target of $15.71 implies further upside potential of 29.4% from current levels.
Related News:
Novavax Up 7% As Pivotal Covid-19 Vaccine Trial Kicks Off In UK
Bausch Scores FDA Approval For Preservative Free Allergy Eyedrop
Aquestive Plunges 37% As FDA Rejects Seizure Drug; Street Stays Bullish









