Incyte (INCY) and MorphoSys (MOR) have announced that the US Food and Drug Administration (FDA) has approved Monjuvi (tafasitamab-cxix) plus lenalidomide for adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) not otherwise specified.
This includes DLBCL arising from low grade lymphoma, and patients who are not eligible for autologous stem cell transplant (ASCT).
DLBCL is the most common type of non-Hodgkin lymphoma in adults, with rapidly growing masses of malignant B-cells in the lymph nodes, spleen, liver, bone marrow or other organs. It is an aggressive disease with one third of patients not responding to initial therapy or relapsing. In the US each year approximately 10,000 patients are diagnosed with relapsed or refractory DLBCL who are not eligible for ASCT.
Monjuvi, a humanized Fc-modified cytolytic CD19 targeting monoclonal antibody, was approved under accelerated approval by the FDA based on overall response rate (ORR). This means that continued approval may require verification in confirmatory trials.
Results from the treatment’s Phase 2 L-MIND study showed an overall response rate (ORR) of 55% (primary endpoint), including a complete response (CR) rate of 37% and a partial response rate (PR) of 18%. The median duration of response (mDOR) was 21.7 months (key secondary endpoint).
The FDA decision represents the first approval of a second-line treatment for adult patients who progressed during or after first-line therapy.
“The approval of Monjuvi brings a new treatment option to patients in dire need across the United States,” cheered Professor Gilles Salles, lead investigator of the L-MIND study. “Today’s FDA decision offers new hope for patients with this aggressive form of DLBCL who progressed during or after first-line therapy.”
Monjuvi is expected to be commercially available in the US shortly, where MorphoSys and Incyte will co-commercialize Monjuvi. Outside the US, Incyte has exclusive commercialization rights.
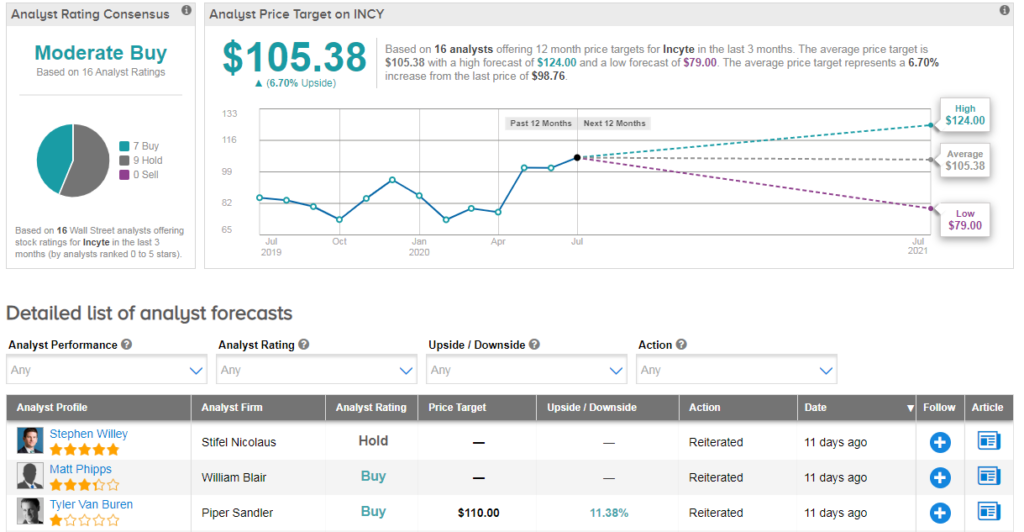
Shares in Incyte are up 13% year-to-date, while MOR has sunk 10%. Looking forward, analysts see 7% further upside potential for Incyte with an average analyst price target of $105. The Street’s consensus on the stock is a cautiously optimistic Moderate Buy.
“Tafasitamab looms on the horizon as another key revenue-driver longer term” wrote Oppenheimer analyst Jay Olson recently. He has a buy rating on the stock and $121 price target (23% upside potential). “We view INCY as well-positioned with cash-generative Jakafi sales and future revenues driven by recent approvals and late-stage assets. Several key catalysts are expected in 2H across the pipeline, among which is the tafasitamab PDUFA in 2L DLBCL” the analyst told investors.
He believes that INCY’s lead marketed product, Jakafi, a JAK-1/2 inhibitor, has significant potential in myelofibrosis (MF) and polycythemia vera (PV) and that the company has a deep and promising pipeline which is currently under-valued. (See INCY stock analysis on TipRanks).
Related News:
GW Pharma Scores New FDA Approval For CBD Drug Epidiolex
AstraZeneca To Pay Up To $6B For Daiichi Cancer Drug Deal
Moderna Could Charge $50-$60 Per Covid-19 Vaccine Course- Report
















