Shares of Immix Biopharma (NASDAQ: IMMX) popped in morning trading on Wednesday, soaring by more than 50% after the biopharma company announced that it had “in-licensed” a BCMA-targeted next-generation CAR-T therapy NXC-201 (formerly HBI0101).
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
The company stated that the Phase 1B clinical trial for NXC-201 in the first 20 patients with relapsed or refractory multiple myeloma indicated an 85% overall response rate (ORR) and 71% showed a complete response/stringent complete response (CR/sCR).
Furthermore, IMMX stated in its press release, “NXC-201 also produced 100% ORR and 100% organ response rate in 4 relapsed/refractory AL Amyloidosis patients.”
IMMX also announced the creation of a wholly-owned subsidiary, Nexcella to look at the development and potential commercialization of NXC-201.
The biopharma company expects that the “formation of Nexcella is expected to have a minimal impact on Immix Biopharma’s financial position, as Nexcella, Inc is expected to be an independently financed company.”
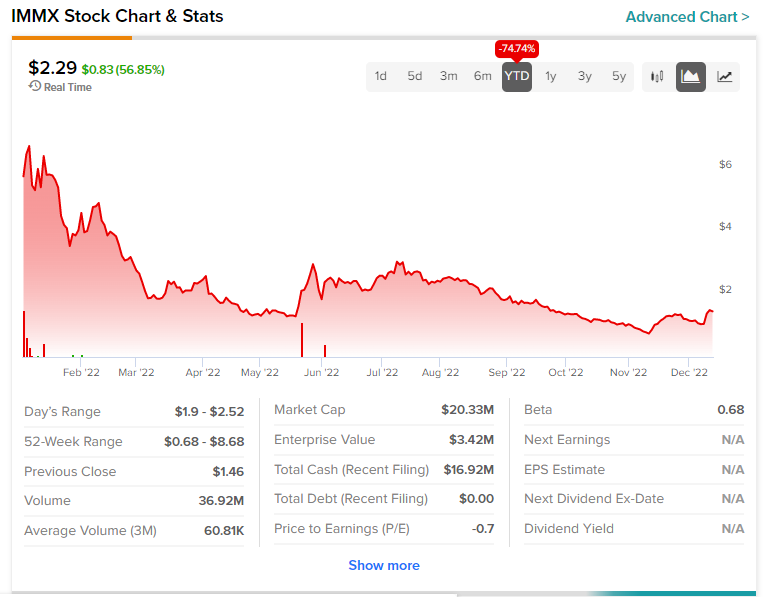
Shares of IMMX have tanked by more than 70% this year.









