Shares of biotechnology company ImmunityBio (NASDAQ:IBRX) are plummeting today after it received a complete response letter (CRL) from the U.S. Food and Drug Administration (FDA) for its biologics license application (BLA) for the Anktiva and Bacillus Calmette Guerin (BCG) combination.
Don't Miss our Black Friday Offers:
- Unlock your investing potential with TipRanks Premium - Now At 40% OFF!
- Make smarter investments with weekly expert stock picks from the Smart Investor Newsletter
The BLA was for the treatment of BCG-unresponsive non-muscle invasive bladder cancer and the FDA has refused approval for the BLA in its current form.
The regulatory body has made recommendations for certain issues including its pre-license inspection of IBRX’s third-party contract manufacturers.
Further, while the FDA has not requested any new clinical studies, it has asked for updated information on the duration of response data for efficacy and made recommendations related to additional chemistry, assays and manufacturing, and controls.
Next, IBRX plans to hold a meeting with the FDA to address the issues raised in the CRL and seek product approval.
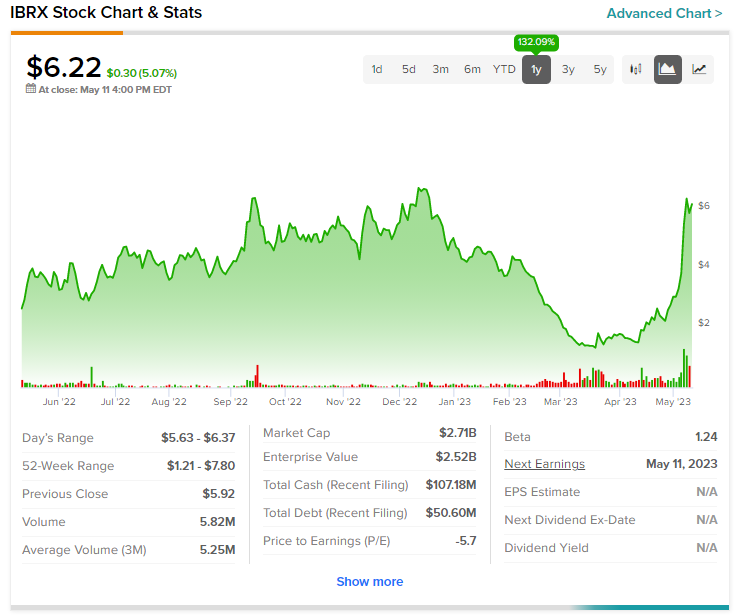
Shares of the company are down nearly 51% at the time of publishing today and short interest in the stock currently stands at about 24.4%.
Read full Disclosure



















