Gilead Sciences (GILD) and Galapagos NV have announced that the European Commission (EC) has granted a marketing authorization for Jyseleca (filgotinib 200 mg and 100 mg tablets), a once-daily, oral, JAK1 inhibitor to treat adults with moderate to severe active rheumatoid arthritis (RA) who have responded inadequately to disease modifying anti-rheumatic drugs (DMARDs).
Discover the Best Stocks and Maximize Your Portfolio:
- See what stocks are receiving strong buy ratings from top-rated analysts.
- Filter, analyze, and streamline your search for investment opportunities with TipRanks’ Stock Screener.
The companies also revealed that the Japanese Ministry of Health, Labour and Welfare has granted regulatory approval for the drug. In Japan, Gilead will hold the marketing authorization and will be responsible for product supply, while Eisai will be responsible for product distribution.
Jyseleca may be used as monotherapy or in combination with methotrexate (MTX).
Almost 3 million people in Europe are living with RA- and without long-term symptom control these people can experience frequent symptom flares and disease progression, significantly impacting their quality of life.
“Despite the availability of existing therapies, new treatment options are still needed to help optimally manage the impact of RA on patients’ daily lives. Jyseleca has demonstrated robust symptom control and prevention of disease progression with a consistent safety profile across the clinical development program,” said Peter C. Taylor of the University of Oxford.
The EC’s decision is supported by data from over 3,500 patients treated with Jyseleca across the Phase 3 FINCH and Phase 2 DARWIN programs. A significantly higher proportion of patients treated with Jyseleca 200 mg plus MTX or other disease-modifying anti-rheumatic drugs achieved low disease activity and/or remission at Weeks 12 and 24 compared with placebo or MTX.
Across the FINCH and DARWIN trials, the most common adverse reactions were nausea, upper respiratory tract infection, urinary tract infection and dizziness.
Gilead and Galapagos NV (GLPG) are collaborative partners in the global development and commercialization of filgotinib in RA and other inflammatory indications. The companies are conducting global studies investigating the potential role of Jyseleca in a variety of diseases, including the Phase 3 SELECTION trial in ulcerative colitis.
Under the collaboration agreement, Galapagos will now receive a milestone payment of $75 million in recognition of the approval of Jyseleca by the European Commission. Shares in Galapagos rose 5% in Friday’s trading.
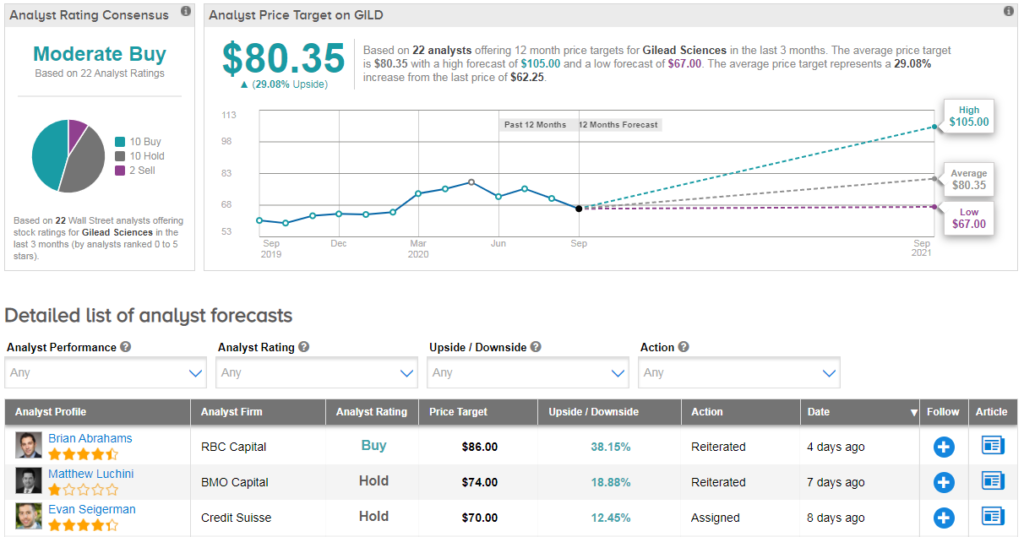
Meanwhile shares in Gilead have declined about 4% year-to-date with the $80.35 average price target indicating 29% upside potential lies ahead in the coming months.
Needham analyst Alan Carr earlier this month reiterated a Hold rating on the stock, citing concerns over revenue growth. “Challenges include competitive environments in HIV, HCV, autoimmune disease, oncology, and cell therapy,” Carr wrote in a note to investors.
The rest of the Street is cautiously optimistic on the stock. The Moderate Buy analyst consensus is based on 10 Buys versus 10 Holds and 2 Sells. (See Gilead stock analysis on TipRanks).
Related News:
J&J Kicks Off Phase 3 Covid-19 Vaccine Trial With Single Dose
RedHill Gets Brazil’s Nod For Covid-19 Study With Opaganib
Teva Launches Two Digital Inhalers For Asthma Patients









