Shares of Gilead Sciences, Inc. (GILD) closed 1.3% higher on Friday after the biopharmaceutical company announced that it submitted a Biologics License Application (BLA) with the U.S. Food and Drug Administration (FDA) for bulevirtide.
If approved, Bulevirtide, an investigational candidate, will be the first-of-its-kind antiviral medicine for the treatment of chronic hepatitis delta virus (HDV) infection in adults with compensated liver disease. It has been granted Breakthrough Therapy and Orphan Drug designations by the FDA.
According to Gilead, the BLA submission is supported by data from completed and ongoing Phase 2 studies and the ongoing Phase 3 MYR301 study, which supports the safety and efficacy of bulevirtide 2 mg once daily after 24 weeks of therapy.
Other Approvals
In Europe, the European Medicines Agency (EMA) has granted Priority Medicines (PRIME) scheme eligibility, and the European Commission has provided Conditional Marketing Authorization for Hepcludex (bulevirtide) as the first-of-its-kind treatment in Europe for adults with the same disease.
Official Comments
The CMO of Gilead, Merdad Parsey, said, “Our goal is to bring safe and effective treatments to people living with the most severe form of chronic viral hepatitis that is associated with rapid progression to serious complications, including fibrosis, cirrhosis, and an increased risk of liver cancer and death.”
“As a leader in hepatology, this filing is the latest milestone in Gilead’s ongoing efforts to address the needs of people living with liver diseases and leverages our deep understanding of chronic viral hepatitis. We look forward to working with the FDA with the goal of bringing this innovation to people living with HDV as quickly as possible,” Parsey added.
See Insiders’ Hot Stocks on TipRanks >>
Wall Street’s Take
On November 18, Wedbush analyst Robert Driscoll maintained a Buy rating on the stock and lifted the price target to $67 (2.5% downside potential) from $52.
Meanwhile, the Street is cautiously optimistic about the stock and has a Moderate Buy consensus rating based on 11 Buys and 5 Holds. The average Gilead price target of $78.86 implies 14.8% upside potential. Shares have gained 14.7% over the past year.
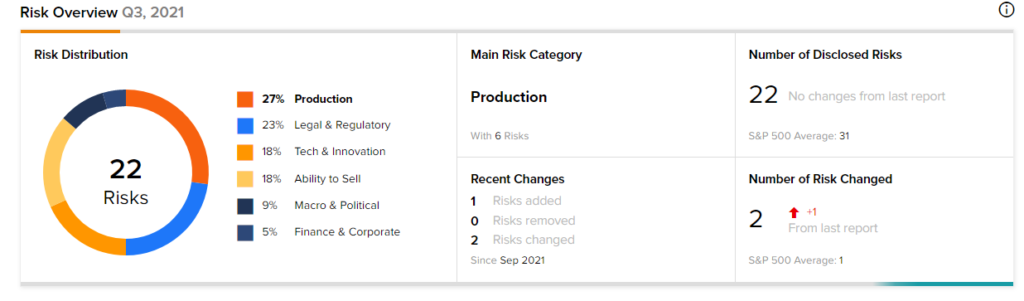
Risk Analysis
According to the new TipRanks’ Risk Factors tool, Gilead is at risk mainly from three factors: Production, Legal and Regulatory, and Tech and Innovation, which contribute 27%, 23%, and 18%, respectively, to the total 22 risks identified for the stock.
Related News:
CVS Health to Commence New Retail Footprint Strategy with Closure of 900 Stores
Clover Health Prices Class A Common Stock Offering Worth $300M
Pfizer Receives EC Approval for XELJANZ
















